
An Introduction to Physical Science
14th Edition
ISBN: 9781305079120
Author: James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher: Brooks Cole
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 6E
Classify each of the following hydrocarbon structural formulas as an
- (a)
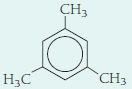
- (b) CH3CH2 CH2CH2CH3
- (c)
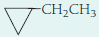
- (d) H─C≡C─CH2CH3
- (e)
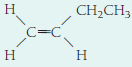
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The cylindrical beam of a 12.7-mW laser is 0.920 cm in diameter. What is the rms value of the electric field?
V/m
Consider a rubber rod that has been rubbed with fur to give the rod a net negative charge, and a glass rod that has been rubbed with silk to give it a net positive charge. After being charged by contact by the fur and silk...?
a. Both rods have less mass
b. the rubber rod has more mass and the glass rod has less mass
c. both rods have more mass
d. the masses of both rods are unchanged
e. the rubber rod has less mass and the glass rod has mroe mass
8) 9)
Chapter 14 Solutions
An Introduction to Physical Science
Ch. 14.1 - Prob. 1PQCh. 14.1 - Prob. 2PQCh. 14.1 - Which of the following two structural formulas is...Ch. 14.2 - Prob. 1PQCh. 14.2 - Prob. 2PQCh. 14.2 - Prob. 14.2CECh. 14.3 - Prob. 1PQCh. 14.3 - Which type of organic material is most used as an...Ch. 14.3 - The octane rating for gasoline assigns a value of...Ch. 14.4 - Prob. 1PQ
Ch. 14.4 - Which types of hydrocarbon derivatives have foul...Ch. 14.4 - Two constitutional isomers of C3H7F exist. Draw...Ch. 14.5 - Prob. 1PQCh. 14.5 - Prob. 2PQCh. 14.5 - Prob. 14.5CECh. 14.6 - Prob. 1PQCh. 14.6 - What is the composition of soap?Ch. 14 - Prob. AMCh. 14 - Prob. BMCh. 14 - Prob. CMCh. 14 - Prob. DMCh. 14 - Prob. EMCh. 14 - Prob. FMCh. 14 - Prob. GMCh. 14 - Prob. HMCh. 14 - Prob. IMCh. 14 - Prob. JMCh. 14 - Prob. KMCh. 14 - Prob. LMCh. 14 - Prob. MMCh. 14 - Prob. NMCh. 14 - Prob. OMCh. 14 - Prob. PMCh. 14 - Prob. QMCh. 14 - Prob. RMCh. 14 - Prob. SMCh. 14 - Prob. TMCh. 14 - Prob. UMCh. 14 - Prob. VMCh. 14 - Prob. WMCh. 14 - Prob. XMCh. 14 - Prob. YMCh. 14 - Prob. ZMCh. 14 - Prob. 1MCCh. 14 - Prob. 2MCCh. 14 - Which of the following is the most common aromatic...Ch. 14 - Prob. 4MCCh. 14 - Prob. 5MCCh. 14 - Prob. 6MCCh. 14 - Which of the following is a correct formula for an...Ch. 14 - Prob. 8MCCh. 14 - Which of these are the best-known synthetic...Ch. 14 - Prob. 10MCCh. 14 - Prob. 11MCCh. 14 - Prob. 12MCCh. 14 - Prob. 1FIBCh. 14 - Prob. 2FIBCh. 14 - Prob. 3FIBCh. 14 - Prob. 4FIBCh. 14 - Prob. 5FIBCh. 14 - A compound with the general formula CnH2n is...Ch. 14 - Prob. 7FIBCh. 14 - Prob. 8FIBCh. 14 - Prob. 9FIBCh. 14 - Prob. 10FIBCh. 14 - Prob. 11FIBCh. 14 - Prob. 12FIBCh. 14 - Prob. 1SACh. 14 - Prob. 2SACh. 14 - Tell the number of covalent bonds formed by an...Ch. 14 - Prob. 4SACh. 14 - Show the Kekul representation and the preferred...Ch. 14 - What structural feature distinguishes an aromatic...Ch. 14 - Give the general molecular formulas for alkanes,...Ch. 14 - Name the first eight members of the alkane series....Ch. 14 - Prob. 9SACh. 14 - Prob. 10SACh. 14 - Prob. 11SACh. 14 - Use both full and condensed structural formulas to...Ch. 14 - Prob. 13SACh. 14 - Name the compound represented by a hexagon and...Ch. 14 - Both ethene and ethyne are often called by their...Ch. 14 - Distinguish between saturated and unsaturated...Ch. 14 - Prob. 17SACh. 14 - Prob. 18SACh. 14 - Give the condensed structural formula for...Ch. 14 - Give the general formula for an alcohol. Name the...Ch. 14 - What characteristic group does an amine contain,...Ch. 14 - Prob. 22SACh. 14 - Prob. 23SACh. 14 - Prob. 24SACh. 14 - Prob. 25SACh. 14 - Prob. 26SACh. 14 - Prob. 27SACh. 14 - Prob. 28SACh. 14 - Prob. 29SACh. 14 - What two simpler sugars combine to form sucrose?...Ch. 14 - Prob. 31SACh. 14 - Prob. 32SACh. 14 - Prob. 33SACh. 14 - Prob. 34SACh. 14 - Prob. 1VCCh. 14 - Prob. 1AYKCh. 14 - You overhear someone comment that a lot of cat...Ch. 14 - Look up the structural formula of aspirin...Ch. 14 - As you take out the garbage one morning, you see...Ch. 14 - Prob. 5AYKCh. 14 - Prob. 6AYKCh. 14 - Prob. 7AYKCh. 14 - Which of these structural formulas is valid, and...Ch. 14 - Prob. 2ECh. 14 - Prob. 3ECh. 14 - Prob. 4ECh. 14 - Classify each of the following hydrocarbon...Ch. 14 - Prob. 7ECh. 14 - Prob. 8ECh. 14 - Prob. 9ECh. 14 - State whether the structural formulas shown in...Ch. 14 - Prob. 11ECh. 14 - Given the IUPAC name, draw the structural formula...Ch. 14 - Prob. 13ECh. 14 - Prob. 14ECh. 14 - Identify each structural formula as belonging to...Ch. 14 - Identify each structural formula as belonging to...Ch. 14 - Prob. 17ECh. 14 - Draw the constitutional isomers for (a) C2H7N...Ch. 14 - Prob. 19ECh. 14 - Acrilan is an addition polymer made from the...Ch. 14 - Prob. 21ECh. 14 - The polyester formed from lactic acid (shown...Ch. 14 - Draw the resulting dipeptide formed from the...Ch. 14 - Draw the resulting dipeptide formed from the...Ch. 14 - Prob. 25ECh. 14 - Prob. 26E
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
The active ingredient in Tylenol and a host of other over-the-counter pain relievers is acetaminophen (C8H9NO2)...
Chemistry: Atoms First
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Lab 8 Part 3 PHET Wave Interface simulation. I am having trouble with this part of the lab.arrow_forwardMick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?arrow_forwardHi, I have canceled, why did you charge me again?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
A Level Physics – Ideal Gas Equation; Author: Atomi;https://www.youtube.com/watch?v=k0EFrmah7h0;License: Standard YouTube License, CC-BY