
Concept explainers
Identify each structural formula as belonging to an
- (a) CH3CH2COOH
- (b)
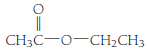
- (c) CH3CH2 CH2OH
- (d) CH3CH2NH2
- (e) CF3CF3
- (f)
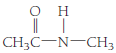
(a)
Answer to Problem 16E
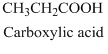
Explanation of Solution
The structural formula belongs to carboxylic acid family as it has
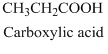
The structural formula of the compound has three carbon atoms longest chain which also includes
Conclusion:
Therefore, structurla formula belongs to carboxylic acid family. The structural formula of the compound is,
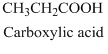
(b)
Answer to Problem 16E
Explanation of Solution
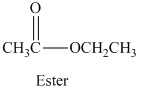
Esters are the organic compounds which are formed by the reaction of alcohols and carboxylic acid reaction, this reaction is also known as esterification reaction. When an alcohol reacts with a carboxylic acid the nucleophilic substitution takes place. The reaction can be written as,
The general molecular formula to identify an ester is
The structural formula of the compound is,

Conclusion:
Therefore structurla formula belongs to an ester family. The structural formula of the compound is,

(c)
Answer to Problem 16E
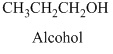
Explanation of Solution
Alcohols: The hydrocarbons in which hydrogen atom is replaced by hydroxy
Methanol is the first member of alcohol family it has
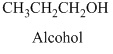
Therefore, this compound belongs to an alcohol family of organic compounds.
Conclusion:
Therefore structurla formula belongs to an alcohol family. The structural formula of the compound is
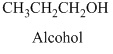
(d)
Answer to Problem 16E
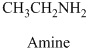
Explanation of Solution
A hydrocarbon in which hydrogen atom is replaced by
Here
Therefore, this given structural formula belongs to an amine family. The structure of the compound is,
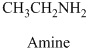
Conclusion:
The structurla formula belongs to an amine family. The structural formula of the compound is
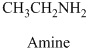
(e)
Answer to Problem 16E
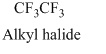
Explanation of Solution
Alkyl halides: When hydrogen atom is replaced by halogen atoms
Here,
The structural formula of the compound is,
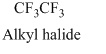
Conclusion:
Therefore, structurla formula belongs to an alkyl halides family. The structural formula of the compound is
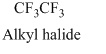
(f)
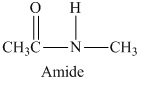
Answer to Problem 16E
The structural formula of the compound is
 c
c
Explanation of Solution
Amides: These organic compounds are synthesized by the condensation reaction of carboxylic acids and amines. The functional group
A tertiary amine does not react with carboxylic acids; hence, no amide formation takes place. However, primary and secondary amines react with carboxylic acid and gives
The given structural formula belongs to the class of amides, which is formed by the reaction of acetic acid with methyl amine. The structural formula of the compound is,

Conclusion:
Therefore, structurla formula belongs to an amide family of organic compounds. The structural formula of the compound is

Want to see more full solutions like this?
Chapter 14 Solutions
An Introduction to Physical Science
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Fundamentals of Physics Extended
Campbell Biology in Focus (2nd Edition)
Laboratory Manual For Human Anatomy & Physiology
General, Organic, and Biological Chemistry - 4th edition
- Mick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?arrow_forwardHi, I have canceled, why did you charge me again?arrow_forwardNo chatgpt pls will upvotearrow_forward
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning


