
PRINCIPLES+REACTIONS
8th Edition
ISBN: 9781337759632
Author: Masterton
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 69QAP
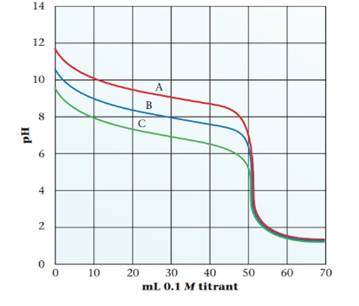
Consider the following titration curves. The solution in the buret is 0.1 M. The solution in the beaker has a volume of 50.0 mL. Answer the following questions.
(a) Is the titrating agent (solution in the buret) an acid or a base?
(b) Which curve shows the titration of the weakest base?
(c) What is the Ka of the conjugate
(d) What is the molarity of the solution in the beaker for curve C?
(e) What is the pH at the equivalence point for curve A?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
oalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.
Write the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcohol
What type of interaction would you expect between the following R groups in the tertiary
structure of a protein?
O
-CH2-CO and -CH2-CH2-CH2-CH2-NH3+
a. disulfide bonds
b. salt bridges
c. hydrogen bonds
HO
abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT
d. hydrophobic interactions
e. peptide bonds
Chapter 14 Solutions
PRINCIPLES+REACTIONS
Ch. 14 - Write a net ionic equation for the reaction...Ch. 14 - Write a net ionic equation for the reaction...Ch. 14 - Write a balanced net ionic equation for the...Ch. 14 - Write a balanced net ionic equation for the...Ch. 14 - Calculate K for the reactions in Question 1.Ch. 14 - Calculate K for the reactions in Question 2.Ch. 14 - Prob. 7QAPCh. 14 - Calculate K for the reactions in Question 4.Ch. 14 - Calculate [H+] and pH in a solution in which...Ch. 14 - Calculate [OH-] and pH in a solution in which the...
Ch. 14 - A buffer is prepared by dissolving 0.0250 mol of...Ch. 14 - A buffer is prepared by dissolving 0.062 mol of...Ch. 14 - A buffer solution is prepared by adding 15.00 g of...Ch. 14 - A buffer solution is prepared by adding 5.50 g of...Ch. 14 - A solution with a pH of 9.22 is prepared by adding...Ch. 14 - An aqueous solution of 0.057 M weak acid, HX, has...Ch. 14 - Which of the following would form a buffer if...Ch. 14 - Which of the following would form a buffer if...Ch. 14 - Calculate the pH of a solution prepared by mixing...Ch. 14 - Calculate the pH of a solution prepared by mixing...Ch. 14 - Calculate the pH of a solution prepared by mixing...Ch. 14 - Calculate the pH of a solution prepared by mixing...Ch. 14 - Consider the weak acids in Table 13.2. Which...Ch. 14 - Prob. 24QAPCh. 14 - A sodium hydrogen carbonate-sodium carbonate...Ch. 14 - You want to make a buffer with a pH of 10.00 from...Ch. 14 - Prob. 27QAPCh. 14 - The buffer capacity indicates how much OH- or H+...Ch. 14 - A buffer is made up of 0.300 L each of 0.500 M...Ch. 14 - A buffer is made up of 239 mL of 0.187 M potassium...Ch. 14 - Enough water is added to the buffer in Question 29...Ch. 14 - Enough water is added to the buffer in Question 30...Ch. 14 - A buffer is prepared in which the ratio [ H2PO4...Ch. 14 - A buffer is prepared using the butyric...Ch. 14 - Blood is buffered mainly by the HCO3 H2CO3 buffer...Ch. 14 - There is a buffer system in blood H2PO4 HPO42 that...Ch. 14 - Given three acid-base indicators—methyl orange...Ch. 14 - Given the acid-base indicators in Question 37,...Ch. 14 - Metacresol purple is an indicator that changes...Ch. 14 - Thymolphthalein is an indicator that changes from...Ch. 14 - When 25.00 mL of HNO3 are titrated with Sr(OH)2,...Ch. 14 - A solution of KOH has a pH of 13.29. It requires...Ch. 14 - A solution consisting of 25.00 g NH4Cl in 178 mL...Ch. 14 - A 50.0-mL sample of NaHSO3 is titrated with 22.94...Ch. 14 - A sample of 0.220 M triethylamine, (CH3CH2)3 N, is...Ch. 14 - A 35.00-mL sample of 0.487 M KBrO is titrated with...Ch. 14 - A 0.4000 M solution of nitric acid is used to...Ch. 14 - A 0.2481 M solution of KOH is used to titrate...Ch. 14 - Consider the titration of butyric acid (HBut) with...Ch. 14 - Morphine, C17H19O3N, is a weak base (K b =7.4107)....Ch. 14 - Consider a 10.0% (by mass) solution of...Ch. 14 - A solution is prepared by dissolving 0.350 g of...Ch. 14 - Prob. 53QAPCh. 14 - Ammonia gas is bubbled into 275 mL of water to...Ch. 14 - For an aqueous solution of acetic acid to be...Ch. 14 - Prob. 56QAPCh. 14 - Prob. 57QAPCh. 14 - Water is accidentally added to 350.00 mL of a...Ch. 14 - A solution of an unknown weak base...Ch. 14 - Consider an aqueous solution of HF. The molar heat...Ch. 14 - Each symbol in the box below represents a mole of...Ch. 14 - Use the same symbols as in Question 61 ( = anion,...Ch. 14 - The following is the titration curve for the...Ch. 14 - Prob. 64QAPCh. 14 - Follow the directions of Question 64. Consider two...Ch. 14 - Prob. 66QAPCh. 14 - Indicate whether each of the following statements...Ch. 14 - Prob. 68QAPCh. 14 - Consider the following titration curves. The...Ch. 14 - Consider the titration of HF (K a=6.7104) with...Ch. 14 - The species called glacial acetic acid is 98%...Ch. 14 - Four grams of a monoprotic weak acid are dissolved...Ch. 14 - Prob. 73QAPCh. 14 - Fifty cm3 of 1.000 M nitrous acid is titrated with...Ch. 14 - A diprotic acid, H2B(MM=126g/moL), is determined...Ch. 14 - Prob. 76QAPCh. 14 - Two students were asked to determine the Kb of an...Ch. 14 - How many grams of NaOH must be added to 1.00 L of...Ch. 14 - How many grams of NaF must be added to 70.00 mL of...Ch. 14 - Prob. 80QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forward
- Draw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forward
- EFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardIf we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardYou need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY