
EBK CHEMISTRY:CENTRAL SCIENCE
14th Edition
ISBN: 9780134554570
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 69E
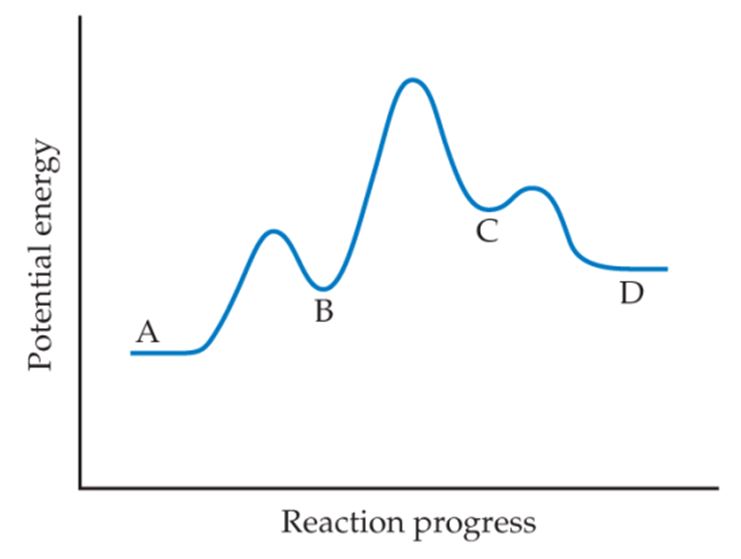
(a) based on the following reaction profile, how many intermediates are formed in the reaction A  D?
D?
(b) How many transition states are there?
(c) Which step is the fastest?
(d) For the reaction A D, is ΔE positive, negative, or zero?
D, is ΔE positive, negative, or zero?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a) A favorable entropy change occurs when ΔS is positive. Does the order of the system increase or decrease when ΔS is positive? (b) A favorable enthalpy change occurs when ΔH is negative. Does the system absorb heat or give off heat when ΔH is negative? (c) Write the relation between ΔG, ΔH, and ΔS. Use the results of parts (a) and (b) to state whether ΔG must be positive or negative for a spontaneous change. For the reaction, ΔG is 59.0 kJ/mol at 298.15 K. Find the value of K for the reaction.
A sample of hydrated magnesium sulfate (MgSO4⋅xH2O) is analyzed using thermogravimetric analysis (TGA). The sample weighs 2.50 g initially and is heated in a controlled atmosphere. As the temperature increases, the water of hydration is released in two stages: (a) The first mass loss of 0.72 g occurs at 150°C, corresponding to the loss of a certain number of water molecules. (b) The second mass loss of 0.90 g occurs at 250°C, corresponding to the loss of the remaining water molecules. The residue is identified as anhydrous magnesium sulfate (MgSO4) Questions: (i) Determine the value of x (the total number of water molecules in MgSO4⋅xH2O) (ii) Calculate the percentage of water in the original sample. Write down the applications of TGA.
The solubility product of iron(III) hydroxide (Fe(OH)3) is 6.3×10−38. If 50 mL of a 0.001 M FeCl3 solution is mixed with 50 mL of a 0.005 M NaOH solution, will Fe(OH)3 precipitate? Show all step-by-step calculations. To evaluate the equilibrium constant, we must express concentrations of solutes in mol/L, gases in bars, and omit solids, liquids, and solvents. Explain why.
Chapter 14 Solutions
EBK CHEMISTRY:CENTRAL SCIENCE
Ch. 14.2 - If the experiment in Figure 14.2 is run for 60 s,...Ch. 14.2 - Prob. 14.1.2PECh. 14.2 - Which of the following could be the instantaneous...Ch. 14.2 - Using Figure 14.3, determine the instantaneous...Ch. 14.2 - At a certain time in a reaction, substance A is...Ch. 14.2 - Prob. 14.3.2PECh. 14.3 - Suppose the rate law for the reaction in this...Ch. 14.3 - Assuming that rate = k[A][B], rank the mixtures...Ch. 14.3 - Prob. 14.5.1PECh. 14.3 - Prob. 14.5.2PE
Ch. 14.3 - Consider the reaction examined above in the Sample...Ch. 14.3 - The following data were measured for the reaction...Ch. 14.4 - At 25 ° C, the decomposition of dinitrogen...Ch. 14.4 - Practice Exercise 2 The decomposition of dimethyl...Ch. 14.4 - Practice Exercise 1 For a certain reaction A ...Ch. 14.4 - Prob. 14.8.2PECh. 14.4 - Practice Exercise 1 We noted in an earlier...Ch. 14.4 - Practice Exercise 2 Using Equation 14.17,...Ch. 14.5 - Practice Exercise 1 This of the following change...Ch. 14.5 - Practice Exercise 2 Rank the rate constants of the...Ch. 14.5 - Practice Exercise 1 Using the data in Sample...Ch. 14.5 - Practice Exercise 2 To one significant figure,...Ch. 14.6 - Practice Exercise 1 Consider the following...Ch. 14.6 - For the reaction Mo(CO)6 +P(CH3)3 Mo(CO)5P(CH3)3...Ch. 14.6 - Practice Exercise 1 Consider the following...Ch. 14.6 - Practice Exercise 2 Consider the following...Ch. 14.6 - Practice Exercise 1 An Alternative two-step...Ch. 14.6 - Prob. 14.14.2PECh. 14.6 - Practice Exercise 1
Consider the...Ch. 14.6 - Prob. 14.15.2PECh. 14 - Prob. 1DECh. 14 - An automotive fuel injector dispenses a fine spray...Ch. 14 - Consider the following graph of the concentration...Ch. 14 - You study the rate of a reaction, measuring both...Ch. 14 - Suppose that for the reaction K+L M, you monitor...Ch. 14 - Prob. 5ECh. 14 - A friend studies a first-order reaction and...Ch. 14 - Prob. 7ECh. 14 - Which of the following linear plots do you expect...Ch. 14 - Prob. 9ECh. 14 - Prob. 10ECh. 14 - The following graph shows two different reaction...Ch. 14 - Prob. 12ECh. 14 - Prob. 13ECh. 14 - Draw a possible transition state for the...Ch. 14 - The following diagram represents an imaginary...Ch. 14 - 14.16 Draw a graph showing the reaction pathway...Ch. 14 - Prob. 17ECh. 14 - 14.18 (a) what are the units usually used to...Ch. 14 - Prob. 19ECh. 14 - A flask is charged with 0.100 mol of A and allowed...Ch. 14 - The isomerization of methyl isontrile (CH3NC) to...Ch. 14 - Prob. 22ECh. 14 - Prob. 23ECh. 14 - For each of the following gas-phase reactions,...Ch. 14 - (a) Consider the combustion of hydrogen, 2H2 (g) +...Ch. 14 - Prob. 26ECh. 14 - A reaction A+B C obeys the following rate law:...Ch. 14 - Prob. 28ECh. 14 - 14.29 The decomposition reaction of N2O5 in carbon...Ch. 14 - Prob. 30ECh. 14 - Prob. 31ECh. 14 - The reaction between ethyl bromide (C2H5Br) and...Ch. 14 - Prob. 33ECh. 14 - The reaction 2ClO2 (aq) + 2OH- (aq) ClO3- (aq) +...Ch. 14 - The following data were measured for the reaction...Ch. 14 - The following data were collected for the rate of...Ch. 14 - Consider the gas-phase reaction between nitric...Ch. 14 - Prob. 38ECh. 14 - Prob. 39ECh. 14 - Prob. 40ECh. 14 - Prob. 41ECh. 14 - Molecular iodine, I2 (g), dissociates into iodine...Ch. 14 - Prob. 43ECh. 14 - Prob. 44ECh. 14 - The reaction SO2Cl2 (g) O2 (g) + Cl2 (g) is first...Ch. 14 - Prob. 46ECh. 14 - Prob. 47ECh. 14 - Prob. 48ECh. 14 - Prob. 49ECh. 14 - Prob. 50ECh. 14 - (a) what factors determine whether a collision...Ch. 14 - (a) in which of the following reactions you expect...Ch. 14 - Calculate the fraction of atoms in a sample of...Ch. 14 - (a) the activation energy for the isomerization of...Ch. 14 - The gas-phase reaction CL (g) + HBr (g) + HCl (g)...Ch. 14 - Prob. 56ECh. 14 - Indicate whether each statement is true or false....Ch. 14 - Indicate whether each statement is true or false....Ch. 14 - Based on their activation energies and energy...Ch. 14 - Prob. 60ECh. 14 - Prob. 61ECh. 14 - Prob. 62ECh. 14 - The rate of the reaction CH3COOC2H5 (aq) + OH- ...Ch. 14 - Prob. 64ECh. 14 - Prob. 65ECh. 14 - Prob. 66ECh. 14 - What is the molecularity of each of the following...Ch. 14 - Prob. 68ECh. 14 - (a) based on the following reaction profile, how...Ch. 14 - Prob. 70ECh. 14 - Prob. 71ECh. 14 - Prob. 72ECh. 14 - The reaction 2NO (g) + CL2 (g) 2NOCl (g) was...Ch. 14 - You have studied the gas-phase oxidation of HBr by...Ch. 14 - Prob. 75ECh. 14 - Prob. 76ECh. 14 - Prob. 77ECh. 14 - Prob. 78ECh. 14 - Prob. 79ECh. 14 - The addition of No accelerates the decomposition...Ch. 14 - 14.81b Many metallic catalysts, particularly the...Ch. 14 - Prob. 82ECh. 14 - When D2 reacts with ethylene (C2H4) in the...Ch. 14 - Prob. 84ECh. 14 - Prob. 85ECh. 14 - The enzyme urease catalyzez the reaction of urea,(...Ch. 14 - Prob. 87ECh. 14 - Prob. 88ECh. 14 - Prob. 89AECh. 14 - Prob. 90AECh. 14 - Prob. 91AECh. 14 - Prob. 92AECh. 14 - Prob. 93AECh. 14 - Prob. 94AECh. 14 - Prob. 95AECh. 14 - Prob. 96AECh. 14 - [14.97]A first order reaction A B has the rate...Ch. 14 - Prob. 98AECh. 14 - Prob. 99AECh. 14 - Prob. 100AECh. 14 - Prob. 101AECh. 14 - Prob. 102AECh. 14 - Cyclopentadiene (C5H6) reacts with itself to form...Ch. 14 - Prob. 104AECh. 14 - At 280C, raw milk sours in 4.0 h but takes 48 h to...Ch. 14 - Prob. 106AECh. 14 - Prob. 107AECh. 14 - Prob. 108AECh. 14 - Prob. 109AECh. 14 - The following mechanism has been proposed for the...Ch. 14 - Prob. 111AECh. 14 - Prob. 112AECh. 14 - Platinum nanoparticles of diameter ~2 nm are...Ch. 14 - 14.114 One of the many remarkable enzymes in the...Ch. 14 - 14.115N Suppose that, in the absence of catalyst,...Ch. 14 - Prob. 116AECh. 14 - Dinitrogen pentoxide (N2O5) decomposes in...Ch. 14 - The reaction between ethyl iodide and hydroxide...Ch. 14 - Prob. 119IECh. 14 - Prob. 120IECh. 14 - Prob. 121IECh. 14 - The rates of many atmospheric reactions are...Ch. 14 - Prob. 123IECh. 14 - Prob. 124IECh. 14 - Prob. 125IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major products of this organic reaction.arrow_forward2. Provide the structure of the major organic product in the following reaction. Pay particular attention to the regio- and stereochemistry of your product. H3CO + H CN Aarrow_forwardPredict the major products of the following organic reaction.arrow_forward
- 1) The isoamyl acetate report requires eight paragraphs - four for comparison of isoamyl alcohol and isoamyl acetate (one paragraph each devoted to MS, HNMR, CNMR and IR) and four for comparison of acetic acid and isoamyl acetate ((one paragraph each devoted to MS, HNMR, CNMR and IR. 2) For MS, the differing masses of molecular ions are a popular starting point. Including a unique fragmentation is important, too. 3) For HNMR, CNMR and IR state the peaks that are different and what makes them different (usually the presence or absence of certain groups). See if you can find two differences (in each set of IR, HNMR and CNMR spectra) due to the presence or absence of a functional group. Include peak locations. Alternatively, you can state a shift of a peak due to a change near a given functional group. Including peak locations for shifted peaks, as well as what these peaks are due to. Ideally, your focus should be on not just identifying the differences but explaining them in terms of…arrow_forwardWhat steps might you take to produce the following product from the given starting material? CI Br Он до NH2 NH2arrow_forward1) The isoamyl acetate report requires eight paragraphs - four for comparison of isoamyl alcohol and isoamyl acetate (one paragraph each devoted to MS, HNMR, CNMR and IR) and four for comparison of acetic acid and isoamyl acetate ((one paragraph each devoted to MS, HNMR, CNMR and IR. 2) For MS, the differing masses of molecular ions are a popular starting point. Including a unique fragmentation is important, too. 3) For HNMR, CNMR and IR state the peaks that are different and what makes them different (usually the presence or absence of certain groups). See if you can find two differences (in each set of IR, HNMR and CNMR spectra) due to the presence or absence of a functional group. Include peak locations. Alternatively, you can state a shift of a peak due to a change near a given functional group. Including peak locations for shifted peaks, as well as what these peaks are due to. Ideally, your focus should be on not just identifying the differences but explaining them in terms of…arrow_forward
- №3 Fill in the below boxes. HN 1. LAH 2. H3O+ NH2arrow_forwardFor the photochemical halogenation reaction below, draw both propagation steps and include the mechanism arrows for each step. H CH ot CH3 CI-CI MM hv of CH H-CI CH3 2nd attempt See Periodic Table See Hint Draw only radical electrons; do not add lone pair electrons. Note that arrows cannot meet in "space," and must end at either bonds or at atoms. 1 i Add the missing curved arrow notation to this propagation step. 20 H ن S F P H CI Br 品arrow_forwardThe radical below can be stabilized by resonance. 4th attempt Draw the resulting resonance structure. DOCEarrow_forward
- Use curved arrows to generate a second resonance form for the allylic radical formed from 2-methyl-2-pentene. 1 Draw the curved arrows that would generate a second resonance form for this radical. D 2 H S F A Бг Iarrow_forwardDraw the resulting product(s) from the coupling of the given radicals. Inlcude all applicable electrons and non-zero formal charges. H.C öö- CH3 2nd attempt +1 : 招 H₂C CH CH₂ See Periodic Table See H H C S F P Br CH₂ Iarrow_forwardPlease, help me out with the calculation, step by step on how to find what's blank with the given information.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Kinetics: Initial Rates and Integrated Rate Laws; Author: Professor Dave Explains;https://www.youtube.com/watch?v=wYqQCojggyM;License: Standard YouTube License, CC-BY