
CONCEPT. INTEG. SCI. -ACCESS W/ ETEXT
3rd Edition
ISBN: 9780135626566
Author: Hewitt
Publisher: INTER PEAR
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 53TE
What are the chemical formula for the following structures?
(a) 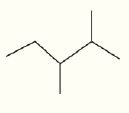 (b)
(b) 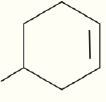 (c)
(c) 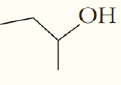 (d)
(d) 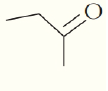
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How can you tell which vowel is being produced here ( “ee,” “ah,” or “oo”)? Also, how would you be able to tell for the other vowels?
You want to fabricate a soft microfluidic chip like the one below. How would you go about
fabricating this chip knowing that you are targeting a channel with a square cross-sectional
profile of 200 μm by 200 μm. What materials and steps would you use and why? Disregard the
process to form the inlet and outlet.
Square Cross Section
1. What are the key steps involved in the fabrication of a semiconductor device.
2. You are hired by a chip manufacturing company, and you are asked to prepare a silicon wafer
with the pattern below. Describe the process you would use.
High Aspect
Ratio
Trenches
Undoped Si Wafer
P-doped Si
3. You would like to deposit material within a high aspect ratio trench. What approach would you
use and why?
4. A person is setting up a small clean room space to carry out an outreach activity to educate high
school students about patterning using photolithography. They obtained a positive photoresist, a
used spin coater, a high energy light lamp for exposure and ordered a plastic transparency mask
with a pattern on it to reduce cost. Upon trying this set up multiple times they find that the full
resist gets developed, and they are unable to transfer the pattern onto the resist. Help them
troubleshoot and find out why pattern of transfer has not been successful.
5. You are given a composite…
Chapter 14 Solutions
CONCEPT. INTEG. SCI. -ACCESS W/ ETEXT
Ch. 14 - How do two structural isomers differ from each...Ch. 14 - How do two structural isomers similar to each...Ch. 14 - What physical property of hydrocarbons is used in...Ch. 14 - What types of hydrocarbons are more abundant in...Ch. 14 - To how many atoms is a saturated carbon atom...Ch. 14 - What is the difference between a saturated...Ch. 14 - How many multiple bonds must a hydrocarbon have in...Ch. 14 - Aromatic compounds contain what kind of ring?Ch. 14 - What is a heteroatom?Ch. 14 - Why do heteroatoms make such a difference in the...
Ch. 14 - How is a heteroatom related to a functional group?Ch. 14 - Why are low-formula-mass alcohols soluble in...Ch. 14 - What distinguishes an alcohol from a phenol?Ch. 14 - What distinguishes an alcohol from an ether?Ch. 14 - Which hetroatom is characteristic of an amine?Ch. 14 - Do amines tend to be acidic, neutral, or basic?Ch. 14 - Are alkaloids found in nature?Ch. 14 - What are some examples of alkaloids?Ch. 14 - Which elements make up the carbonyl group?Ch. 14 - How are ketones and aldehydes related to each...Ch. 14 - How are amides and carboxylic acids related to...Ch. 14 - From what naturally occurring compound is aspirin...Ch. 14 - What happens to the double bond of a monomer that...Ch. 14 - What is released in the formation of a...Ch. 14 - Why is plastic wrap made of polyvinylidene...Ch. 14 - Prob. 26RCCCh. 14 - In the lock-and-key model, is a drug viewed as the...Ch. 14 - What holds a drug to its receptor site?Ch. 14 - Which fits better into the opioid receptor...Ch. 14 - How does the effect of a drug wear off?Ch. 14 - Prob. 34TCCh. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Prob. 39TECh. 14 - What property of carbon allows for the formation...Ch. 14 - Prob. 41TECh. 14 - Draw all the structural isomers for hydrocarbons...Ch. 14 - How many structural isomers are shown here? .Ch. 14 - Prob. 44TECh. 14 - How many different conformation are possible for...Ch. 14 - Prob. 46TECh. 14 - The temperatures in a fractionating tower at an...Ch. 14 - There are five atoms in the methane molecule, CH4....Ch. 14 - Compared to lighter hydrocarbons, do heavier...Ch. 14 - What do these two structures have in common?Ch. 14 - With four unpaired valence electrons, how can...Ch. 14 - What do the compounds cyclopropane and propene...Ch. 14 - What are the chemical formula for the following...Ch. 14 - Remember that carbon-carbon single bonds can...Ch. 14 - Which of the structures shown in the previous...Ch. 14 - Why are there so many different organic compounds?Ch. 14 - Identify the following functional groups-amide,...Ch. 14 - What must be added to a double bond to transform...Ch. 14 - What do phenols and carboxylic acids have in...Ch. 14 - What is the difference between a ketone and an...Ch. 14 - Prob. 61TECh. 14 - What do alcohols, phenols, and ethers have in...Ch. 14 - Prob. 63TECh. 14 - What is the percent volume of water in 80- proof...Ch. 14 - One of the skin-irritating components of poison...Ch. 14 - Prob. 66TECh. 14 - Prob. 67TECh. 14 - A common inactive ingredient in products such as...Ch. 14 - A common inactive ingredient in products such as...Ch. 14 - The phosphoric acid salt of caffeine has the...Ch. 14 - Prob. 71TECh. 14 - In water, does the following molecule act as an...Ch. 14 - Prob. 73TECh. 14 - The amino acid lysine is shown here. What...Ch. 14 - Why does the carbon of the carbonyl usually have a...Ch. 14 - Prob. 76TECh. 14 - Suggest an explanation for why aspirin has a sour...Ch. 14 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 14 - What products are formed upon the reaction of...Ch. 14 - The disodium salt of ethylenediaminetetraacetic...Ch. 14 - Prob. 81TECh. 14 - Which is better for you: a drug that is a natural...Ch. 14 - Naloxone is a molecule that binds to the opioid...Ch. 14 - What use might there be for Naloxone?Ch. 14 - Rank the following from least ideal to most ideal...Ch. 14 - Why are plastics generally so inexpensive?Ch. 14 - Would you expect polypropylene to be denser or...Ch. 14 - Hydrocarbons release a lot of energy when ignited....Ch. 14 - The polymer styrene-butadiene rubber SBR, shown...Ch. 14 - Citral and camphor are both 10 carbon odoriferous...Ch. 14 - Many of the natural product molecules synthesized...Ch. 14 - The solvent diethyl ether can be mixed with water...Ch. 14 - Alkaloid salts are not very soluble in the organic...Ch. 14 - Go online and look up the total synthesis of the...Ch. 14 - Medicines, such as pain relievers and...Ch. 14 - Why does the melting point of hydrocarbons get...Ch. 14 - Prob. 2RATCh. 14 - Which contains more hydrogen atoms a five-carbon...Ch. 14 - Prob. 4RATCh. 14 - Why might a high-formula-mass alcohol be insoluble...Ch. 14 - Alkaloids salts are not very soluble in the...Ch. 14 - Explain why caprylic acid, CH3(CH2)6COOH,...Ch. 14 - How many oxygen atoms are bonded to the carbon of...Ch. 14 - Prob. 9RATCh. 14 - Prob. 10RAT
Additional Science Textbook Solutions
Find more solutions based on key concepts
You microscopically examine scrapings from a case of Acan-thamoeba keratitis. You expect to see a. nothing. b. ...
Microbiology: An Introduction
26. A 10 kg crate is placed on a horizontal conveyor belt. The materials are such that and .
a. Draw a free-...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Using the South Atlantic as an example, label the beginning of the normal polarity period C that began 2 millio...
Applications and Investigations in Earth Science (9th Edition)
Explain all answer clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desig...
Cosmic Perspective Fundamentals
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Where is transitional epithelium found and what is its importance at those sites?
Anatomy & Physiology (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Two complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find r and θ for (z1-z∗2)/z1+z2∗. Find r and θ for (z1−z2∗)z1z2∗ Please show all stepsarrow_forwardAn electromagnetic wave is traveling through vacuum in the positive x direction. Its electric field vector is given by E=E0sin(kx−ωt)j^,where j^ is the unit vector in the y direction. If B0 is the amplitude of the magnetic field vector, find the complete expression for the magnetic field vector B→ of the wave. What is the Poynting vector S(x,t), that is, the power per unit area associated with the electromagnetic wave described in the problem introduction? Give your answer in terms of some or all of the variables E0, B0, k, x, ω, t, and μ0. Specify the direction of the Poynting vector using the unit vectors i^, j^, and k^ as appropriate. Please explain all stepsarrow_forwardAnother worker is performing a task with an RWL of only 9 kg and is lifting 18 kg, giving him an LI of 2.0 (high risk). Questions:What is the primary issue according to NIOSH?Name two factors of the RWL that could be improved to reduce risk.If the horizontal distance is reduced from 50 cm to 30 cm, how does the HM change and what effect would it have?arrow_forward
- Two complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find r and θ for z1z2∗. Find r and θ for z1/z2∗? Find r and θ for (z1−z2)∗/z1+z2∗. Find r and θ for (z1−z2)∗/z1z2∗ Please explain all steps, Thank youarrow_forwardAn ac series circuit consists of a voltage source of frequency 60 Hz and voltage amplitude V, a 505-Ω resistor, and a capacitor of capacitance 7.2 μF. What must be the source voltage amplitude V for the average electrical power consumed in the resistor to be 236 W? There is no inductance in the circuit.arrow_forwardAn L−R−C series circuit has R= 280 Ω . At the frequency of the source, the inductor has reactance XLL= 905 Ω and the capacitor has reactance XC= 485 Ω . The amplitude of the voltage across the inductor is 445 V . What is the amplitude of the voltage across the resistor and the capacitor? What is the voltage amplitude of the source? What is the rate at which the source is delivering electrical energy to the circuit?arrow_forward
- A 0.185 H inductor is connected in series with a 98.5 Ω resistor and an ac source. The voltage across the inductor is vL=−(12.5V)sin[(476rad/s)t]vL. Derive an expression for the voltage vR across the resistor. Express your answer in terms of the variables L, R, VL (amplitude of the voltage across the inductor), ω, and t. What is vR at 2.13 ms ? Please explain all stepsarrow_forwardA worker lifts a box under the following conditions:Horizontal distance (H): 30 cmInitial height (V): 60 cmVertical travel (D): 50 cmTorso rotation (A): 30°Frequency: 3 times/minute for 1 hourGrip: Good Question:What is the RWL for this task?What does this value mean in terms of occupational safety?arrow_forwardCan someone helparrow_forward
- Can someone help mearrow_forward3. Four identical small masses are connected in a flat perfect square. Rank the relative rotational inertias (IA, IB, IC) about the three axes of rotation shown. Axes A and B are in the plane of the square, and axis C is perpendicular to the plane, through mass m1. ΙΑ IB m2 m1 m3 Ic m4 (a) IAarrow_forwardConsider the circuit shown in the figure below. (Assume L = 5.20 m and R2 = 440 Ω.) (a) When the switch is in position a, for what value of R1 will the circuit have a time constant of 15.4 µs? (b) What is the current in the inductor at the instant the switch is thrown to position b?arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning


Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY