
Concept explainers
(a)
Interpretation:
The statement “ethyl butanoate is an ether” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(a)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate is an ester of ethanol and butanoic acid.
Explanation of Solution
Ethyl butanoate is an ester, which can be synthesized from butanoic acid and ethanol.
The
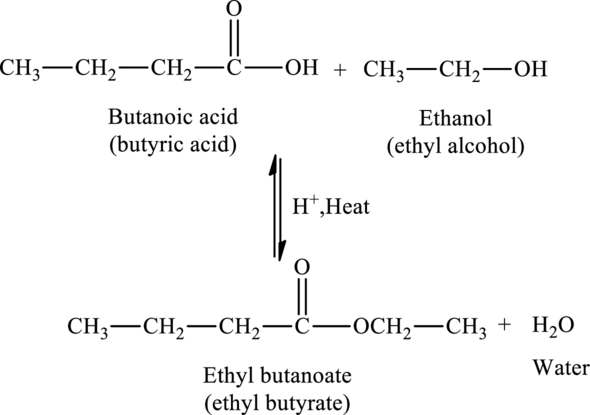
The statement “ethyl butanoate is an ether” is false because ethyl butanoate is an ester of ethanol and butanoic acid.
(b)
Interpretation:
The statement “ethyl butanoate has a higher melting point than hexanoic acid” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(b)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate is lower melting point than hexanoic acid.
Explanation of Solution
Hexanoic acid is a
Carboxylic acids have strong intermolecular hydrogen bonding with each other and strong dipole-dipole attractions.
The presence of polar carboxyl group and intermolecular hydrogen bonding makes these acids have higher boiling points. The presence of dimers increases the strength of the van der Waals dispersion forces, which makes them have high boiling points.
Ester does not engage in hydrogen bonding with each other, but they form hydrogen bonds with water.
Hence, the statement ethyl butanoate has a higher melting point than hexanoic acid” is false because hexanoic acid has strong intermolecular hydrogen bonding and strong dipole-dipole attractions when compared to ethyl butanoate.
(c)
Interpretation:
The statement “ethyl butanoate forms hydrogen bonds with water” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(c)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Esters can form hydrogen bonds with water. The hydrogen bonds are formed through their oxygen atoms to the hydrogen atoms of water. Thus, esters are slightly soluble in water.
(d)
Interpretation:
The statement “ethyl butanoate is nonpolar” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(d)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate is slightly polar.
Explanation of Solution
The structure of ethyl butanoate is,
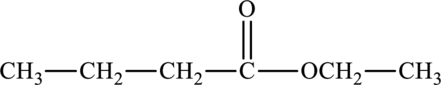
The carbonyl group present in ethyl butanoate is polar in nature and thereby can participate in dipole-dipole attractions. Therefore, ethyl butanoate is polar in nature.
The statement “ethyl butanoate is nonpolar” is false due to the presence of polar carbonyl group in them; ethyl butanoate is polar in nature.
(e)
Interpretation:
The statement “ethyl butanoate has a molecular formula of
(e)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate has a molecular formula of
Explanation of Solution
The structure of ethyl butanoate is,
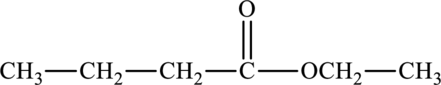
From the structure of ethyl butanoate, it can be seen that ethyl butanoate comprises of six carbon atoms, twelve hydrogen atoms and two oxygen atoms. Hence the molecular formula of ethyl butanoate is
The statement “ethyl butanoate has a molecular formula of
(f)
Interpretation:
The statement “ethyl butanoate has a higher boiling point than hexanol” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(f)
Answer to Problem 4MCP
The given statement is false because hexanol has higher boiling point than ethyl butanoate. The lack of strong intermolecular hydrogen bonding makes ethyl butanoate have less boiling point.
Explanation of Solution
Alcohols have strong intermolecular hydrogen bonding. The
Ester does not engage in hydrogen bonding with each other, but they form hydrogen bonds with water.
The absence of strong intermolecular hydrogen bonding makes ethyl butanoate have lesser boiling point than hexanol. Hence, the statement “ethyl butanoate has a higher boiling point than hexanol” is false.
(g)
Interpretation:
The statement “ethyl butanoate is a liquid at room temperature” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(g)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Ethyl butanoate is clear colorless liquid with a pineapple-like odor. It is less dense than water and insoluble in water. It has a fruity odor, similar to pineapple and is a key ingredient used as flavor enhancer in processed orange juices. Hence, the statement “ethyl butanoate is a liquid at room temperature” is true.
(h)
Interpretation:
The statement “ethyl butanoate is a water soluble” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(h)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate is insoluble in water.
Explanation of Solution
Ethyl butanoate is very hydrophobic molecule and it is insoluble in water.
Ethyl butanoate forces themselves between water molecules; they break the strong hydrogen bonds between water molecules without replacing them. This makes the process energetically less profitable and so solubility decreases. As the chain lengths increase, the hydrocarbon parts of the ester molecules start to get in the way.
Hence, the statement “ethyl butanoate is a water soluble” is false because it insoluble in water due to its hydrophobic nature.
(i)
Interpretation:
The statement “Ethyl butanoate has a carboxyl
(i)
Answer to Problem 4MCP
The given statement is false because ethyl butanoate has a carbonyl functional group.
Explanation of Solution
Esters are carboxylic acid derivatives. The carbonyl group of the ester is polar and can participate in dipole-dipole attractions.
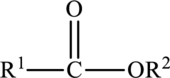
In an ester, the second oxygen atom is bonded to another carbon atom.
A carboxyl group consists of both polar carbonyl and hydroxyl functional groups.
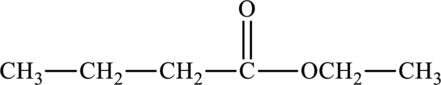
The structure of ethyl butanoate is,
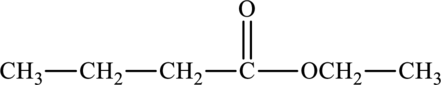
Ethyl butanoate shows the presence of carbonyl group and not carboxyl group.
Hence, the statement “Ethyl butanoate has a carboxyl functional group” is false because it consists of polar carbonyl group.
(j)
Interpretation:
The statement “ethyl butanoate is a structural isomer of hexanoic acid” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(j)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Structural isomers are those with same molecular formula but differ in arrangement of atoms.
The molecular formula of ethyl butanoate is
The molecular formula of hexanoic acid is
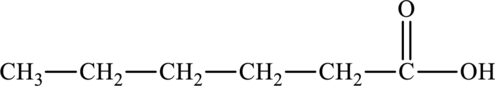
Both these are structural isomers with each other and hence, the statement “ethyl butanoate is a structural isomer of hexanoic acid” is true.
Want to see more full solutions like this?
Chapter 14 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 2.2 mol of NaOH is added to 1.0 L of a 1.4M HF solution. acids: П bases: Х other: ☐ ப acids: 0.51 mol of KOH is added to 1.0 L of a solution that is bases: 1.3M in both HF and NaF. other: ☐ 00. 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2O4 (g) 2NO2 (g) AG⁰ = 5.4 kJ Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2O4 tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. yes no 0.42 atm ☑ 5 0/5 ? مله Ararrow_forwardHomework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forward
- Identifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forwardHighlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward
- € + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forwardDraw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forwardEpoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward
- 03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forwardYou may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





