
Conceptual Integrated Science
3rd Edition
ISBN: 9780135197394
Author: Hewitt, Paul G., LYONS, Suzanne, (science Teacher), Suchocki, John, Yeh, Jennifer (jennifer Jean)
Publisher: PEARSON EDUCATION (COLLEGE)
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 43TE
How many structural isomers are shown here?
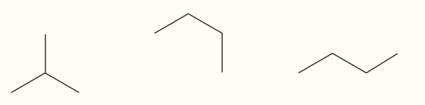 .
.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
no ai please
A block of mass m₁ = 1.85 kg and a block of mass m₂
is 0.360 for both blocks.
=
m
M, R
m2
Ꮎ
5.90 kg are connected by a massless string over a pulley in the shape of a solid disk having a mass of M = 10.0 kg. The fixed, wedge-shaped ramp makes an angle of 0 = 30.0° as shown in the figure. The coefficient of kinetic friction
(a) Determine the acceleration of the two blocks. (Enter the magnitude of the acceleration.)
x m/s²
(b) Determine the tensions in the string on both sides of the pulley.
left of the pulley
× N
right of the pulley
X N
Enter a number.
What is the error determined by the 2/3 rule?
Chapter 14 Solutions
Conceptual Integrated Science
Ch. 14 - How do two structural isomers differ from each...Ch. 14 - How do two structural isomers similar to each...Ch. 14 - What physical property of hydrocarbons is used in...Ch. 14 - What types of hydrocarbons are more abundant in...Ch. 14 - To how many atoms is a saturated carbon atom...Ch. 14 - What is the difference between a saturated...Ch. 14 - How many multiple bonds must a hydrocarbon have in...Ch. 14 - Aromatic compounds contain what kind of ring?Ch. 14 - What is a heteroatom?Ch. 14 - Why do heteroatoms make such a difference in the...
Ch. 14 - How is a heteroatom related to a functional group?Ch. 14 - Why are low-formula-mass alcohols soluble in...Ch. 14 - What distinguishes an alcohol from a phenol?Ch. 14 - What distinguishes an alcohol from an ether?Ch. 14 - Which hetroatom is characteristic of an amine?Ch. 14 - Do amines tend to be acidic, neutral, or basic?Ch. 14 - Are alkaloids found in nature?Ch. 14 - What are some examples of alkaloids?Ch. 14 - Which elements make up the carbonyl group?Ch. 14 - How are ketones and aldehydes related to each...Ch. 14 - How are amides and carboxylic acids related to...Ch. 14 - From what naturally occurring compound is aspirin...Ch. 14 - What happens to the double bond of a monomer that...Ch. 14 - What is released in the formation of a...Ch. 14 - Why is plastic wrap made of polyvinylidene...Ch. 14 - Prob. 26RCCCh. 14 - In the lock-and-key model, is a drug viewed as the...Ch. 14 - What holds a drug to its receptor site?Ch. 14 - Which fits better into the opioid receptor...Ch. 14 - How does the effect of a drug wear off?Ch. 14 - Prob. 34TCCh. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank these hydrocarbons in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Rank the organic molecules in order of increasing...Ch. 14 - Prob. 39TECh. 14 - What property of carbon allows for the formation...Ch. 14 - Prob. 41TECh. 14 - Draw all the structural isomers for hydrocarbons...Ch. 14 - How many structural isomers are shown here? .Ch. 14 - Prob. 44TECh. 14 - How many different conformation are possible for...Ch. 14 - Prob. 46TECh. 14 - The temperatures in a fractionating tower at an...Ch. 14 - There are five atoms in the methane molecule, CH4....Ch. 14 - Compared to lighter hydrocarbons, do heavier...Ch. 14 - What do these two structures have in common?Ch. 14 - With four unpaired valence electrons, how can...Ch. 14 - What do the compounds cyclopropane and propene...Ch. 14 - What are the chemical formula for the following...Ch. 14 - Remember that carbon-carbon single bonds can...Ch. 14 - Which of the structures shown in the previous...Ch. 14 - Why are there so many different organic compounds?Ch. 14 - Identify the following functional groups-amide,...Ch. 14 - What must be added to a double bond to transform...Ch. 14 - What do phenols and carboxylic acids have in...Ch. 14 - What is the difference between a ketone and an...Ch. 14 - Prob. 61TECh. 14 - What do alcohols, phenols, and ethers have in...Ch. 14 - Prob. 63TECh. 14 - What is the percent volume of water in 80- proof...Ch. 14 - One of the skin-irritating components of poison...Ch. 14 - Prob. 66TECh. 14 - Prob. 67TECh. 14 - A common inactive ingredient in products such as...Ch. 14 - A common inactive ingredient in products such as...Ch. 14 - The phosphoric acid salt of caffeine has the...Ch. 14 - Prob. 71TECh. 14 - In water, does the following molecule act as an...Ch. 14 - Prob. 73TECh. 14 - The amino acid lysine is shown here. What...Ch. 14 - Why does the carbon of the carbonyl usually have a...Ch. 14 - Prob. 76TECh. 14 - Suggest an explanation for why aspirin has a sour...Ch. 14 - Benzaldehyde is a fragrant oil. If stored in an...Ch. 14 - What products are formed upon the reaction of...Ch. 14 - The disodium salt of ethylenediaminetetraacetic...Ch. 14 - Prob. 81TECh. 14 - Which is better for you: a drug that is a natural...Ch. 14 - Naloxone is a molecule that binds to the opioid...Ch. 14 - What use might there be for Naloxone?Ch. 14 - Rank the following from least ideal to most ideal...Ch. 14 - Why are plastics generally so inexpensive?Ch. 14 - Would you expect polypropylene to be denser or...Ch. 14 - Hydrocarbons release a lot of energy when ignited....Ch. 14 - The polymer styrene-butadiene rubber SBR, shown...Ch. 14 - Citral and camphor are both 10 carbon odoriferous...Ch. 14 - Many of the natural product molecules synthesized...Ch. 14 - The solvent diethyl ether can be mixed with water...Ch. 14 - Alkaloid salts are not very soluble in the organic...Ch. 14 - Go online and look up the total synthesis of the...Ch. 14 - Medicines, such as pain relievers and...Ch. 14 - Why does the melting point of hydrocarbons get...Ch. 14 - Prob. 2RATCh. 14 - Which contains more hydrogen atoms a five-carbon...Ch. 14 - Prob. 4RATCh. 14 - Why might a high-formula-mass alcohol be insoluble...Ch. 14 - Alkaloids salts are not very soluble in the...Ch. 14 - Explain why caprylic acid, CH3(CH2)6COOH,...Ch. 14 - How many oxygen atoms are bonded to the carbon of...Ch. 14 - Prob. 9RATCh. 14 - Prob. 10RAT
Additional Science Textbook Solutions
Find more solutions based on key concepts
In your own words, briefly distinguish between relative dates and numerical dates.
Applications and Investigations in Earth Science (9th Edition)
Draw the enol tautomers for each of the following compounds. For compounds that have more than one enol tautome...
Organic Chemistry (8th Edition)
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
4. A photographer uses his camera, whose lens has a 50 mm focal length, to focus on an object 2.0 m away. He th...
College Physics: A Strategic Approach (3rd Edition)
Which one of the following is not a fuel produced by microorganisms? a. algal oil b. ethanol c. hydrogen d. met...
Microbiology: An Introduction
Which of the following factors would tend to increase membrane fluidity? A. a greater proportion of unsaturated...
Campbell Biology in Focus (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Your colleague gives you a sample that are supposed to consist of Pt-Ni nanoparticles, TiO2 nanorod arrays, and SiO2 monolith plates (see right panel schematic). The bimetallic Pt-Ni nanoparticles are expected to decorate on the side surfaces of the aligned TiO2 nanorod arrays. These aligned TiO2 nanoarrays grew on the flat SiO2 monolith. Let's assume that the sizes of the Pt-Ni nanoparticles are > 10 nm. We further assume that you have access to a modern SEM that can produce a probe size as small as 1 nm with a current as high as 1 nA. You are not expected to damage/destroy the sample. Hint: keep your answers concise and to the point. TiO₂ Nanorods SiO, monolith a) What do you plan to do if your colleague wants to know if the Pt and Ni formed uniform alloy nanoparticles? (5 points) b) If your colleague wants to know the spatial distribution of the PtNi nanoparticles with respect to the TiO2 nanoarrays, how do you accomplish such a goal? (5 points) c) Based on the experimental results…arrow_forwardFind the current in 5.00 and 7.00 Ω resistors. Please explain all reasoningarrow_forwardFind the amplitude, wavelength, period, and the speed of the wave.arrow_forward
- A long solenoid of length 6.70 × 10-2 m and cross-sectional area 5.0 × 10-5 m² contains 6500 turns per meter of length. Determine the emf induced in the solenoid when the current in the solenoid changes from 0 to 1.5 A during the time interval from 0 to 0.20 s. Number Unitsarrow_forwardA coat hanger of mass m = 0.255 kg oscillates on a peg as a physical pendulum as shown in the figure below. The distance from the pivot to the center of mass of the coat hanger is d = 18.0 cm and the period of the motion is T = 1.37 s. Find the moment of inertia of the coat hanger about the pivot.arrow_forwardReview Conceptual Example 3 and the drawing as an aid in solving this problem. A conducting rod slides down between two frictionless vertical copper tracks at a constant speed of 3.9 m/s perpendicular to a 0.49-T magnetic field. The resistance of th rod and tracks is negligible. The rod maintains electrical contact with the tracks at all times and has a length of 1.4 m. A 1.1-Q resistor is attached between the tops of the tracks. (a) What is the mass of the rod? (b) Find the change in the gravitational potentia energy that occurs in a time of 0.26 s. (c) Find the electrical energy dissipated in the resistor in 0.26 s.arrow_forward
- A camera lens used for taking close-up photographs has a focal length of 21.5 mm. The farthest it can be placed from the film is 34.0 mm. (a) What is the closest object (in mm) that can be photographed? 58.5 mm (b) What is the magnification of this closest object? 0.581 × ×arrow_forwardGiven two particles with Q = 4.40-µC charges as shown in the figure below and a particle with charge q = 1.40 ✕ 10−18 C at the origin. (Note: Assume a reference level of potential V = 0 at r = ∞.) Three positively charged particles lie along the x-axis of the x y coordinate plane.Charge q is at the origin.Charge Q is at (0.800 m, 0).Another charge Q is at (−0.800 m, 0).(a)What is the net force (in N) exerted by the two 4.40-µC charges on the charge q? (Enter the magnitude.) N(b)What is the electric field (in N/C) at the origin due to the two 4.40-µC particles? (Enter the magnitude.) N/C(c)What is the electrical potential (in kV) at the origin due to the two 4.40-µC particles? kV(d)What If? What would be the change in electric potential energy (in J) of the system if the charge q were moved a distance d = 0.400 m closer to either of the 4.40-µC particles?arrow_forward(a) Where does an object need to be placed relative to a microscope in cm from the objective lens for its 0.500 cm focal length objective to produce a magnification of -25? (Give your answer to at least three decimal places.) 0.42 × cm (b) Where should the 5.00 cm focal length eyepiece be placed in cm behind the objective lens to produce a further fourfold (4.00) magnification? 15 × cmarrow_forward
- In a LASIK vision correction, the power of a patient's eye is increased by 3.10 D. Assuming this produces normal close vision, what was the patient's near point in m before the procedure? (The power for normal close vision is 54.0 D, and the lens-to-retina distance is 2.00 cm.) 0.98 x marrow_forwardDon't use ai to answer I will report you answerarrow_forwardA shopper standing 2.00 m from a convex security mirror sees his image with a magnification of 0.200. (Explicitly show on paper how you follow the steps in the Problem-Solving Strategy for mirrors found on page 1020. Your instructor may ask you to turn in this work.) (a) Where is his image (in m)? (Use the correct sign.) -0.4 m in front of the mirror ▾ (b) What is the focal length (in m) of the mirror? -0.5 m (c) What is its radius of curvature (in m)? -1.0 marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning



Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY