
Concept explainers
If myosin V moved more like an “inchworm” (meaning it dragged its rear filament-binding domain forward, but not in a hand-over-hand fashion), how would a graph of spot movement over time differ from the one shown in Figure 14A-2b?
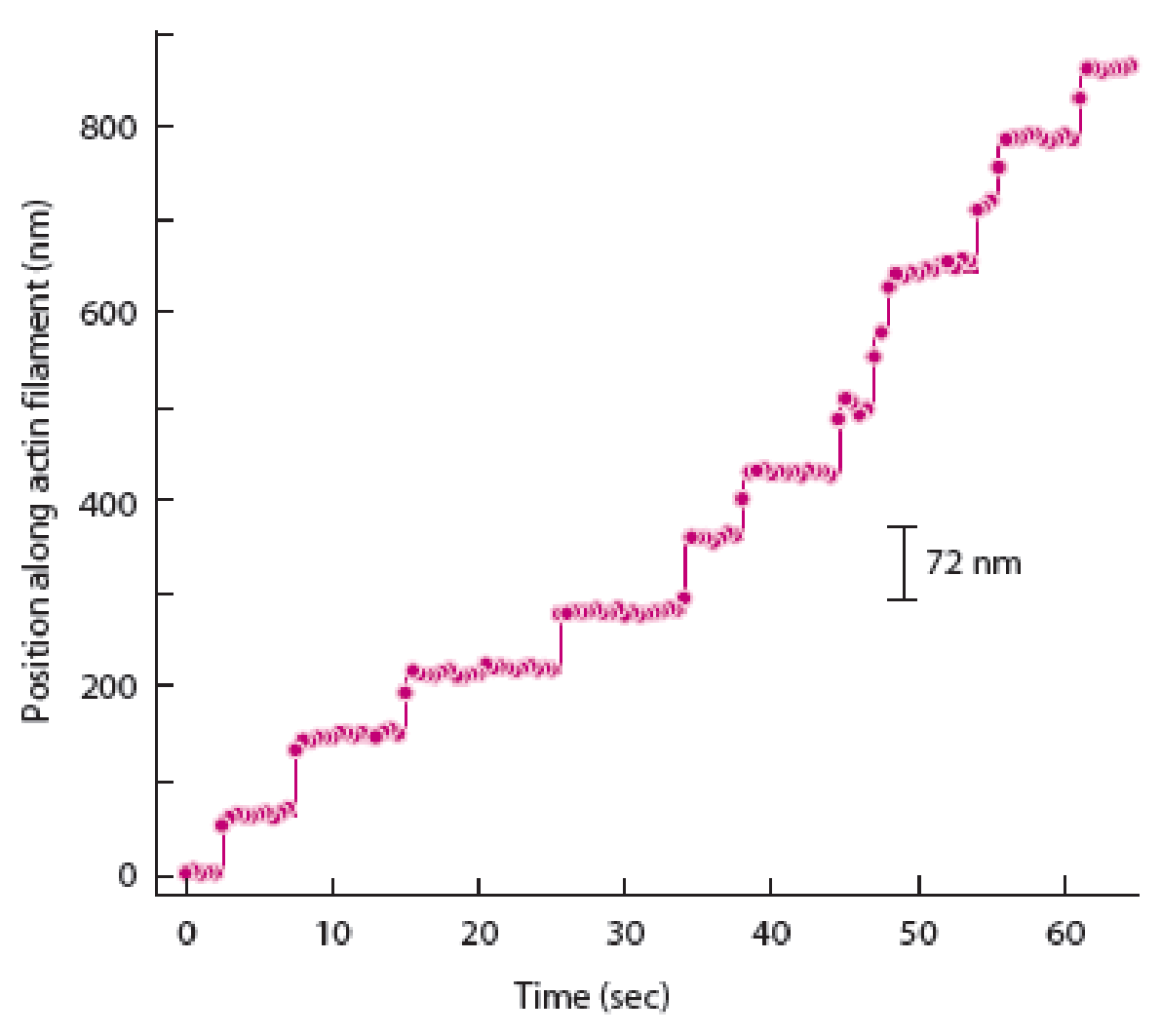
To determine: The way in which graph of spot movement over time differs from the graph shown in figure 14A-2b given in the textbook when myosin V moved like an inchworm.
Introduction: Myosin protein V is an important protein which is involved in the transports of various types of intracellular cargo along with actin proteins. These myosin proteins are involved in the transport of various membrane-bound organelles and show two alternating types of mechanisms for movement. One method is the hand-over-hand method and another method is the inch worm model.
Explanation of Solution
Hand-over-hand mechanism of myosin V movement states that the trailing head would move past the stationary forward head by an approximate distance of 74 nanometers to reach a new actin binding site. In contrast to this, the inch-worm model suggests that both heads would advance or move forward only by 37 nanometers during the step. Both trailing and leading heads do not undergo the process of position exchange during the inch worm model.
The best way to determine the difference between the hand-over-hand model and inch-worm models is to strictly follow the displacement of one of myosin V heads. Therefore, a graph of spot movement over time will differ in case of distance as 74 nanometers (position along actin filament) will be shown in the hand-over-hand model and 35 nanometers will be shown in inchworm model of spot movement.
Want to see more full solutions like this?
Chapter 14 Solutions
Becker's World of the Cell (9th Edition)
- Not part of a graded assignment, from a past midtermarrow_forwardNoggin mutation: The mouse, one of the phenotypic consequences of Noggin mutationis mispatterning of the spinal cord, in the posterior region of the mouse embryo, suchthat in the hindlimb region the more ventral fates are lost, and the dorsal Pax3 domain isexpanded. (this experiment is not in the lectures).a. Hypothesis for why: What would be your hypothesis for why the ventral fatesare lost and dorsal fates expanded? Include in your answer the words notochord,BMP, SHH and either (or both of) surface ectoderm or lateral plate mesodermarrow_forwardNot part of a graded assignment, from a past midtermarrow_forward
- Explain in a flowcharts organazing the words down below: genetics Chromosomes Inheritance DNA & Genes Mutations Proteinsarrow_forwardplease helparrow_forwardWhat does the heavy dark line along collecting duct tell us about water reabsorption in this individual at this time? What does the heavy dark line along collecting duct tell us about ADH secretion in this individual at this time?arrow_forward
 Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning





