
Concept explainers
Answers to all problems are at the end οΓthis book. Detailed solutions are available in the Student Solutions Manual. Study Guide, and Problems Book.
Characterizing a Covalent Enzyme Inhibitor Tosyl-L-phenylalanme cfaloromethyl
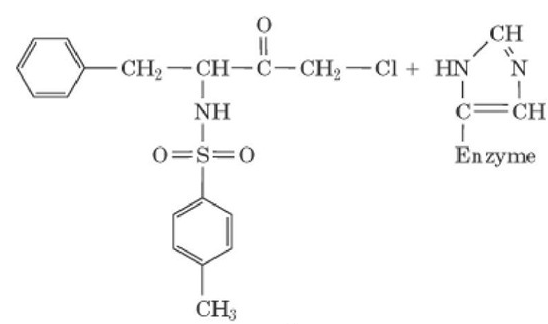
- Propose a mechanism for the inactivation reaction, indicating the structure of the produce(s).
- State why this inhibitor is specific tor cJiymotrypsin.
- Propose a reagent based on the structure of TPCK that might be an effective inhibitor of trypsin.
a.
To propose: The mechanism for the inactivation reaction which indicates the structure of the products.
Introduction:
Covalent enzyme inhibitor like TPCK is slowly reversible or totally irreversible. In many cases, these inhibitors are used to validate the pathway or target. There are targeted covalent enzyme inhibitors that have a slow offset, high binding efficiency as well as high potency to bind, increased selectivity decreased propensity for target-based drug resistance and prolonged pharmacodynamic effect.
Explanation of Solution
Chymotrypsin undergoes covalent inactivation reaction by nucleophilic attack on the
So, the mechanism of inactivation reaction is given below:

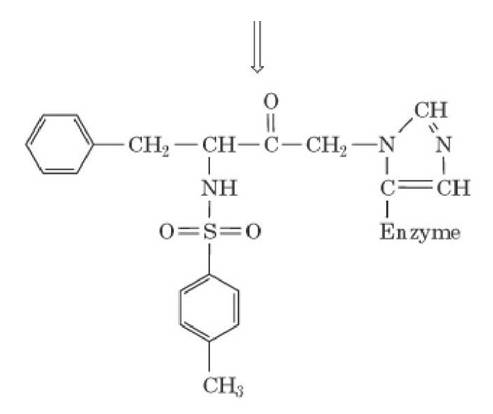
Hence, from the above discussion, it is clear that chymotrypsin undergoes covalent inhibition reaction.
b.
To propose: The reason for this inhibitor to be specific for chymotrypsin.
Introduction:
Covalent enzyme inhibitor like TPCK is slowly reversible or totally irreversible. In many cases, these inhibitors are used to validate the pathway or target. There are targeted covalent enzyme inhibitors that have a slow offset, high binding efficiency as well as high potency to bind, increased selectivity decreased propensity for target-based drug resistance and prolonged pharmacodynamic effects. Chymotrypsin plays an important part in the breakdown of proteins which is called proteolysis. The pancreas synthesizes the chymotrypsin. It cleaves the peptide amino bonds to digest the proteins.
Explanation of Solution
The inhibitor is chymotrypsin specific because the chloromethyl keto group reaches the active site by the hydrophobic reactions of chymotrypsin and the phenyl group of TPCK.
Hence, from the above discussion, it is clear that the inhibitor is chymotrypsin specific.
c.
To identify: A reagent that can be an effective inhibitor of Trypsin.
Introduction:
Chymotrypsin plays an important part in the breakdown of proteins which is called proteolysis. The pancreas synthesizes the chymotrypsin. It cleaves the peptide amino bonds to digest the proteins.
Explanation of Solution
The phenylalanine residue of TPCK has to be replaced with lysine or arginine to produce the specific reagents for the enzyme Trypsin.
Hence, from the above discussion, it is clear that the residual portion of TPCK only produces the specific reagent for Trypsin.
Want to see more full solutions like this?
Chapter 14 Solutions
BIOCHEM. II-EBOOK ACCESS>CUSTOM<
- 7. A solution of aniline in diethyl ether is added to a separatory funnel. Which ONE of the following aqueous solutions will remove aniline from the ether layer (and transfer it to the aqueous layer) if it is added to the separatory funnel and the funnel is shaken? A) aqueous NH3 B) aqueous Na2CO3 C) aqueous HCl D) aqueous NaOH E) aqueous CH3COONa (sodium acetate)arrow_forward11. Which of the following choices is the best reagent to use to perform the following conversion? A) 1. LiAlH4 2. H₂O B) HCI, H₂O D) NaOH, H₂O E) Na№3 ? 'N' 'N' C) 1. CH3MgBr 2. HCI, H₂Oarrow_forwardNH2 ΝΗΣ NH₂ NH2 NH2 A OCH3 NO₂ B C D E 1. Which one of the five compounds above is the strongest base? 2. Which one of the five compounds above is the second-strongest base? (That is, which one is next-to-strongest base?) 3. Which one of the five compounds above is the weakest base? 4. Which one of the five compounds above is the second-weakest base? (That is, which one is next-to-weakest base?) NO2arrow_forward
- Draw a diagram to demonstrate 3' to 5' exonuclease activity?arrow_forward1. Write the piece of mRNA that would code for the amino acid sequence NH3 - met - tyr - cys- his- CO2. Label the 5' and 3' ends. (diagram attached) 2.arrow_forwardYou were tasked with purifying an untagged transcription factor (molecular weight 65,000Da, isoelectric point unknown) from a contaminant Protein A (molecular weight 50,000 Da, isoelectric point 5.0). You were also instructed to use the protein for crystallization studies after purification.i) In the initial purification step, which type of chromatography should you attempt? Explain your choice and specify the requirements of the buffer solution you should use. ii) Analyzing the proteins recovered from step i) using SDS-PAGE, you still observed a faint band of Protein A in addition to the transcription factor band. Given the limited time available for further purification, you must choose ONE appropriate chromatography method to maximize the chances of separating the two proteins as well as your crystallization studies. Provide a detailed explanation of your selection and the techniques/strategies you would employ to achieve this.arrow_forward
- You were given a mixture of two proteins with different isoelectric points and molecular weights:• Protein X: pI 4.2, MW 42,000• Protein Y: pI 9.8, MW 90,000Using a Tris-glycine discontinuous native gel (pH8.3) electrophoresis system with a running gel of 12%, only a single band was observed upon protein staining after electrophoresis. Explain the observed result and discuss possible factors affecting protein migration in this system.arrow_forwardThe standard cost of Product B manufactured by Oriole Company includes 3.5 units of direct materials at $5.40 per unit. During June, 27,300 units of direct materials are purchased at a cost of $5.15 per unit, and 27,300 units of direct materials are used to produce 7,600 units of Product B. (a) Compute the total materials variance and the price and quantity variances. Total materials variance Materials price variance Materials quantity variance $ (b) Compute the total materials variance and the price and quantity variances, assuming the purchase price is $6.35 and the quantity purchased and used is 26,300 units. Total materials variance Materials price variance Materials quantity variance $arrow_forwardThe pyruvate dehydrogenase complex catalyzes the oxidative decarboxylation of pyruvate to form acetyl CoA. E₁, E2, and E3 are abbreviations for the enzymes of the complex. Classify the enzyme names, prosthetic groups, and reactions as E1, E2, or E3. E₁ E2 Answer Bank E3 transfer of electrons to FAD and then to NAD+ transfer of acetyl group to coenzyme A formation of hydroxyethyl-TPP hydroxyethyl group transferred to lipoamide thiamine pyrophosphate (TPP) FAD lipoamide dihydrolipoyl transacetylase pyruvate dehydrogenase dihydrolipoyl dehydrogenasearrow_forward
- Patients with pyruvate dehydrogenase deficiency show high levels of lactic acid in the blood. However, in some cases, treatment with dichloroacetate (DCA), which inhibits the kinase associated with the pyruvate dehydrogenase complex, lowers lactic acid levels. How does DCA act to stimulate pyruvate dehydrogenase activity? DCA activates pyruvate dehydrogenase kinase. DCA increases phosphorylation levels of pyruvate dehydrogenase. DCA inhibits pyruvate dehydrogenase kinase. ODCA activates pyruvate dehydrogenase phosphatase. What does this suggest about pyruvate dehydrogenase activity in patients who respond to DCA? The pyruvate dehydrogenase complex is active only when phosphorylated by the kinase. The pyruvate dehydrogenase complex is active only in the presence of the kinase. The pyruvate dehydrogenase complex is completely inactive. The pyruvate dehydrogenase complex displays some residual activity.arrow_forwardThe reduced coenzymes generated by the citric acid cycle donate electrons in a series of reactions called the electron-transport chain. The energy from the electron-transport chain is used for oxidative phosphorylation. Which compounds donate electrons to the electron- transport chain? H₂O NADH பப NAD+ ATP ADP FADH₂ FAD Which compounds are the final products of the electron-transport chain and oxidative phosphorylation? H₂O NADH NAD+ ΠΑΤΡ Π ADP FADH₂ FAD Which compound is the final electron acceptor in the electron-transport chain? Оно NADH NAD+ ATP ADP FADH₂ FADarrow_forwardHexokinase in red blood cells has a Michaelis constant (KM) of approximately 50 μM. Because life is hard enough as it is, let's assume that hexokinase displays Michaelis-Menten kinetics. What concentration of blood glucose yields an initial velocity (V) equal to 90% of the maximal velocity (Vmax)? [glucose] = What does the calculated substrate concentration at 90% Vmax tell you if normal blood glucose levels range between approximately 3.6 and 6.1 mM? Hexokinase operates near Vmax only when glucose levels are low. Hexokinase normally operates far below Vmax. Hexokinase operates near Vmax only when glucose levels are high. Hexokinase normally operates near Vmax mMarrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
