
Concept explainers
(a)
Interpretation:
The neutralization reaction of pentanoic acid with sodium hydroxide has to be written.
Concept Introduction:
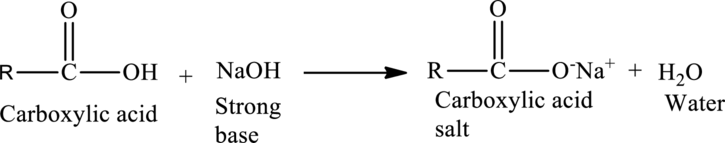
Acid-Base Reactions of Carboxylic acids:
When a strong base is reacted with a
The general reaction is given as,
(b)
Interpretation:
The neutralization reaction of pentanoic acid with potassium hydroxide has to be written.
Concept Introduction:
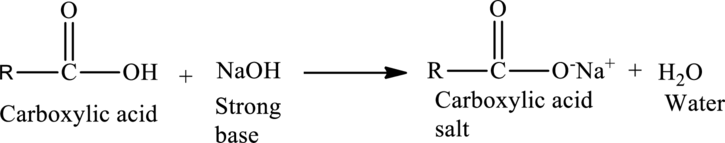
Acid-Base Reactions of Carboxylic acids:
When a strong base is reacted with a carboxylic acid, neutralization occurs. The acid protons are removed by the
The general reaction is given as,
(c)
Interpretation:
The neutralization reaction of pentanoic acid with calcium hydroxide has to be written.
Concept Introduction:
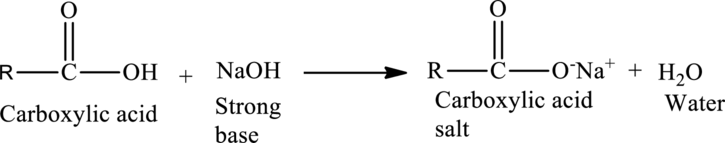
Acid-Base Reactions of Carboxylic acids:
When a strong base is reacted with a carboxylic acid, neutralization occurs. The acid protons are removed by the
The general reaction is given as,
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Connect 2-Year Online Access for General, Organic, and Biochemistry
- Please correct answer and don't use hand ratingarrow_forwardThe SN 1 mechanism starts with the rate-determining step which is the dissociation of the alkyl halide into a carbocation and a halide ion. The next step is the rapid reaction of the carbocation intermediate with the nucleophile; this step completes the nucleophilic substitution stage. The step that follows the nucleophilic substitution is a fast acid-base reaction. The nucleophile now acts as a base to remove the proton from the oxonium ion from the previous step, to give the observed product. Draw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all nonzero formal charges. Cl: Add/Remove step G Click and drag to start drawing a structure.arrow_forwardPlease correct answer and don't use hand ratingarrow_forward
- A monochromatic light with a wavelength of 2.5x10-7m strikes a grating containing 10,000 slits/cm. Determine the angular positions of the second-order bright line.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Us the reaction conditions provided and follow the curved arrow to draw the resulting structure(s). Include all lone pairs and charges as appropriate. H :I H 0arrow_forwardPlease correct answer and don't use hand ratingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





