
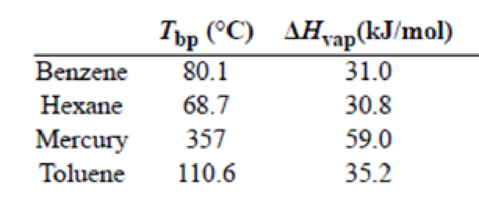
(a) Trouton’s rule states that the ratio of the molar heat of vaporization of a liquid (ΔHvap) to its boiling point in kelvins is approximately 90 J/K · mol. Use the following data to show that this is the case and explain why Trouton’s rule holds true.
(b) Use the values in Table 12.5 to calculate the same ratio for ethanol and water. Explain why Trouton’s rule does not apply to these two substances as well as it does to other liquids.
(a)
Interpretation:
To verify Trouton's rule for the given set of substances.
Concept Introduction:
According to Trouton's rule for, various liquids attheir boiling point the change in entropy value will be the same. The ratio of enthalpy of vaporization and boiling temperature of various liquids has the same value.
Where,
Answer to Problem 14.47QP
Trouton's rule is verified for benzene, hexane, mercury and toluene. All the four substances have
Explanation of Solution
To record the given data
To verify Trouton's rule for benzene
Trouton's rule can be verified by plugging in the values of
Since the value is approximately equal to
To verify Trouton's rule for hexane
Trouton's rule can be verified by plugging in the values of
Since the value is approximately equal to
To verify Trouton's rule for mercury
Trouton's rule can be verified by plugging in the values of
Since the value is approximately equal to
To verify Trouton's rule for Toluene
Trouton's rule can be verified by plugging in the values of
Since the value is approximately equal to
(b)
Interpretation:
To verify Trouton's rule for the given set of substances.
Concept Introduction:
According to Trouton's rule for, various liquids attheir boiling point the change in entropy value will be the same. The ratio of enthalpy of vaporization and boiling temperature of various liquids has the same value.
Where,
Answer to Problem 14.47QP
Trouton's rule is not obeyed by water and ethanol due to the high entropy of vaporization.
Explanation of Solution
To record the given data
To verify Trouton's rule for ethanol
Trouton's rule can be verified by plugging in the values of
Since, the value is greater than
To verify Trouton's rule for water
Trouton's rule can be verified by plugging in the values of
Since the value is greater than
To explain the reason for the deviation from hydrogen bond in case of water and ethanol.
For water and ethanol there exist strong hydrogen bonding attraction in the liquid state. Hydrogen bonding interaction decreases the entropy in the system. In gaseous state the hydrogen bonding interaction becomes weaker and the molecules will have more randomness in the system. The entropy of vaporization will be high. This is the reason why water and ethanol is showing larger deviation from Trouton's rule.
Want to see more full solutions like this?
Chapter 14 Solutions
Chemistry: Atoms First V1
- Calculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.arrow_forwardGiven the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.arrow_forwardThe decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.arrow_forward
- 2. Add the following group of numbers using the correct number of significant figures for the answer. Show work to earn full credit such as rounding off the answer to the correct number of significant figures. Replace the question marks with the calculated answers or write the calculated answers near the question marks. 10916.345 37.40832 5.4043 3.94 + 0.0426 ? (7 significant figures)arrow_forwardThe emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.arrow_forwardIndicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.arrow_forward
- How can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forwardUsing Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forward
- The molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forwardBA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





