
The dissociation of molecular iodine into iodine atoms is represented as
At 1000 K, the equilibrium constant Kc for the reaction is 3.80 × 10−5. Suppose you start with 0.0456 mole of I2 in a 2.30-L flask at 1000 K. What are the concentrations of the gases at equilibrium?
Interpretation:
The equilibrium concentration of hydrogen and iodine gas has to be calculated.
Concept Introduction:
Equilibrium concentration: If Kc and the initial concentration for a reaction and calculate for both equilibrium concentration, and using the (ICE) chart and equilibrium constant and derived changes in respective reactants and products.
Equilibrium constant: Concentration of the products to the respective molar concentration of reactants it is called equilibrium constant. If the K value is less than one the reaction will move to the left side and the K values is higher (or) greater than one the reaction will move to the right side of reaction.
Heterogeneous equilibrium: This equilibrium reaction does not depend on the amounts of pure solid and liquid present, in other words heterogeneous equilibrium, substances are in different phases.
Kp and Kc: This equilibrium constants of gaseous mixtures, these difference between the two constants is that Kc is defined by molar concentrations, whereas Kp is defined by the partial pressures of the gasses inside a closed system.
Vaporized equilibrium: This conversion of liquid in gaseous phase is known as vaporization process. At starting the rate of condensation is less than the rate of evaporation but as evaporation continues the concentration of gaseous molecule in the vapour phase increase.
Answer to Problem 14.44QP
Explanation of Solution
To find: The each reactant product equilibrium concentration should be identified given the gas phase reaction.
Write and Analyze the given gas phase chemical equilibrium reaction.
The given equilibrium reaction has a homogenous process, then the equilibrium constant can also be represented by Kp, were the Kp represents partial pressure. Then the product molecule partial pressure 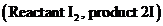 is derived in step-2.
is derived in step-2.
To find: Calculate equilibrium concentration (Kp) values for given the statement of equilibrium reaction.
Calculate and analyze the (Kp) values at
We derived here (Kp) values of (I2) dissociation reaction
First we derived the initial concentration of (I2) is
We consider the equilibrium expression in terms of the equilibrium concentration.
The obtained second (x) values are negative concentration, this physically impossible so we omitted this values. First (x) value is correct one.
The given iodine dissociation equilibrium reaction the respective reactant to give the two moles of products in the gas phase and this equilibrium reaction expression contains single conditions like gases phase, the equilibrium constant can also be represented by Kp, were the “P” partial pressure. The each molar concentration values are Kp derived given the gas phase reaction at
The molar concentration (M) values are derived given the iodine
Want to see more full solutions like this?
Chapter 14 Solutions
CHEMISTRY (LL) W/CNCT >BI<
- Zeroth Order Reaction In a certain experiment the decomposition of hydrogen iodide on finely divided gold is zeroth order with respect to HI. 2HI(g) Au H2(g) + 12(9) Rate = -d[HI]/dt k = 2.00x104 mol L-1 s-1 If the experiment has an initial HI concentration of 0.460 mol/L, what is the concentration of HI after 28.0 minutes? 1 pts Submit Answer Tries 0/5 How long will it take for all of the HI to decompose? 1 pts Submit Answer Tries 0/5 What is the rate of formation of H2 16.0 minutes after the reaction is initiated? 1 pts Submit Answer Tries 0/5arrow_forwardangelarodriguezmunoz149@gmail.com Hi i need help with this question i am not sure what the right answers are.arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Don't used hand raitingarrow_forwardDon't used Ai solutionarrow_forwardSaved v Question: I've done both of the graphs and generated an equation from excel, I just need help explaining A-B. Below is just the information I used to get the graphs obtain the graph please help. Prepare two graphs, the first with the percent transmission on the vertical axis and concentration on the horizontal axis and the second with absorption on the vertical axis and concentration on the horizontal axis. Solution # Unknown Concentration (mol/L) Transmittance Absorption 9.88x101 635 0.17 1.98x101 47% 0.33 2.95x101 31% 0.51 3.95x10 21% 0.68 4.94x10 14% 24% 0.85 0.62 A.) Give an equation that relates either the % transmission or the absorption to the concentration. Explain how you arrived at your equation. B.) What is the relationship between the percent transmission and the absorption? C.) Determine the concentration of the ironlll) salicylate in the unknown directly from the graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight…arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





