
Concept explainers
(a)
Interpretation:
Whether the given molecule is
Concept introduction:
The molecules, to be aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic due to lack of planarity.
Explanation of Solution
The given structure is:
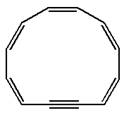
The molecule has total
The molecule is determined as nonaromatic based on the structure planarity.
(b)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic due to lack of planarity.
Explanation of Solution
The given structure is:
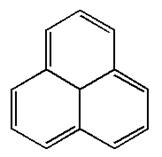
The molecule has total
The molecule is determined as nonaromatic based on the structure planarity.
(c)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is aromatic as it is planar, has cyclic conjugation, and obeys Hückel’s rule.
Explanation of Solution
The given structure is:
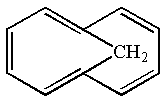
The given molecule is planar and has total
The molecule is determined as aromatic based on the Hückel’s rule.
(d)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic due non cyclic conjugated system.
Explanation of Solution
The given structure is:
![]()
The molecule has a conjugated system of total
The molecule is determined as nonaromatic based on the non cyclic structure which lacks cyclic conjugation.
(e)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is aromatic as it is planar, has cyclic conjugation, and obeys Hückel’s rule.
Explanation of Solution
The given structure is:
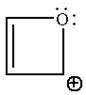
The molecule is planar and has fully cyclic conjugated system of total
The molecule is determined as aromatic based on planarity, fully conjugated system, and having Hückel’s rule of
(f)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic as it is not conjugated and does not obey Hückel’s rule.
Explanation of Solution
The given structure is:
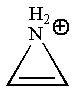
The molecule has only
The molecule is determined as nonaromatic based on the non-conjugated system of
(g)
Interpretation:
Whether the given molecule is aromatic, antiaromatic, or nonaromatic, is to be determined.
Concept introduction:
The molecules, to be an aromatic, must obey Hückel’s rule of
Answer to Problem 14.41P
The given molecule is nonaromatic as it has no cyclic conjugated system.
Explanation of Solution
The given structure is:
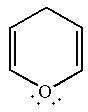
One of the lone pair on the oxygen atom participates in the resonance, thus, the molecule has
The molecule is determined as nonaromatic based on non-conjugated system of
Want to see more full solutions like this?
Chapter 14 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forward
- Draw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forwardChoose the right answerarrow_forward8. What is the major product of the following reaction? KMnO4 b a TOH OH OH C d OH "OH HO OH OHarrow_forward
- Choose the right answerarrow_forward3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward
- 6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forwardWhat is the skeletal structure of the product of the following organic reaction?arrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

