
Chemistry: Atoms First
3rd Edition
ISBN: 9781259923142
Author: Burdge
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 14.2VC
Now consider the reaction
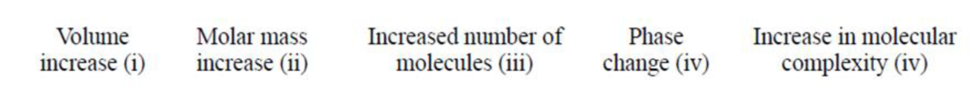
(a) i and iii
(b) I, ii, and iii
(c) i, iv, and v
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The representation of a one-dimensional velocity distribution function for a gas, as the temperature increases:a) it becomes more flattenedb) the maximum occurs for vi = 0 m/sExplain it.
The velocity distribution function of gas moleculesa) is used to measure their velocity, since the small size of gas molecules means that it cannot be measured in any other wayb) is only used to describe the velocity of particles if their density is very high.c) describes the probability that a gas particle has a velocity in a given interval of velocities
Explain why in the representation of a one-dimensional velocity distribution function for a particular gas, the maximum occurs for vi = 0 m/s.
Chapter 14 Solutions
Chemistry: Atoms First
Ch. 14.3 - Determine the change in entropy for 1.0 mole of an...Ch. 14.3 - Determine the change in entropy (Ssys) for the...Ch. 14.3 - To what fraction of its original volume must a...Ch. 14.3 - From the standard entropy values in Appendix 2,...Ch. 14.3 - Prob. 2PPACh. 14.3 - In each of the following reactions, there is one...Ch. 14.3 - For each reaction shown in the diagrams, indicate...Ch. 14.3 - For each process, determine the sign of S for the...Ch. 14.3 - Prob. 3PPACh. 14.3 - Make a qualitative prediction of the sign of Hsoln...
Ch. 14.3 - Consider the gas-phase reaction of A2 (blue) and...Ch. 14.3 - Prob. 14.3.1SRCh. 14.3 - For which of the following chemical reactions is S...Ch. 14.3 - Prob. 14.3.3SRCh. 14.4 - Determine if each of the following is a...Ch. 14.4 - For each of the following, calculate Suniv and...Ch. 14.4 - (a) Calculate Suniv and determine if the reaction...Ch. 14.4 - The following table shows the signs of Ssys,...Ch. 14.4 - Using data from Appendix calculate S (in J/K mol)...Ch. 14.4 - Prob. 14.4.2SRCh. 14.4 - Prob. 14.4.3SRCh. 14.4 - Prob. 14.4.4SRCh. 14.4 - Prob. 14.4.5SRCh. 14.5 - According to Table 14 4, a reaction will be...Ch. 14.5 - A reaction will be spontaneous only at low...Ch. 14.5 - Given that the reaction 4Fe(s) + 3O2(g) + 6H2O(l) ...Ch. 14.5 - Prob. 5PPCCh. 14.5 - Prob. 14.6WECh. 14.5 - Prob. 6PPACh. 14.5 - For each reaction, determine the value of Gf that...Ch. 14.5 - Prob. 6PPCCh. 14.5 - Prob. 14.7WECh. 14.5 - Prob. 7PPACh. 14.5 - Prob. 7PPBCh. 14.5 - Prob. 7PPCCh. 14.5 - Prob. 14.5.1SRCh. 14.5 - Prob. 14.5.2SRCh. 14.5 - Prob. 14.5.3SRCh. 14 - Using Gf values from Appendix 2, calculate the...Ch. 14 - Prob. 14.2KSPCh. 14 - Using Grxnvalues from Appendix 2, calculate the...Ch. 14 - Prob. 14.4KSPCh. 14 - Explain what is meant by a spontaneous process....Ch. 14 - Which of the following processes are spontaneous...Ch. 14 - Prob. 14.3QPCh. 14 - Prob. 14.4QPCh. 14 - Prob. 14.5QPCh. 14 - Prob. 14.6QPCh. 14 - Prob. 14.7QPCh. 14 - Consider two gas samples at STP: one consisting of...Ch. 14 - Now consider the reaction F2(g)2F(g)at constant...Ch. 14 - Which of the following best describes why entropy...Ch. 14 - Which of the following best explains why entropy...Ch. 14 - How does the entropy of a system change for each...Ch. 14 - How does the entropy of a system change for each...Ch. 14 - Predict whether the entropy change is positive or...Ch. 14 - Prob. 14.11QPCh. 14 - Calculate Ssys for (a) the isothermal expansion of...Ch. 14 - Calculate Ssys for (a) the isothermal compression...Ch. 14 - Using the data in Appendix 2, calculate the...Ch. 14 - Using the data in Appendix 2, calculate the...Ch. 14 - For each pair of substances listed here, choose...Ch. 14 - Arrange the following substances (1 mole each) in...Ch. 14 - State the second law of thermodynamics in words,...Ch. 14 - State the third law of thermodynamics in words,...Ch. 14 - Calculate Ssurr for each of the reactions in...Ch. 14 - Calculate Ssurr for each of the reactions in...Ch. 14 - Using data from Appendix 2, calculate Srxn and...Ch. 14 - Using data from Appendix 2, calculate Srxn and...Ch. 14 - When a folded protein in solution is heated to a...Ch. 14 - Define free energy. What are its units?Ch. 14 - Why is it more convenient to predict the direction...Ch. 14 - What is the significance of the sign of Gsys?Ch. 14 - From the following combinations of H and S,...Ch. 14 - Prob. 14.29QPCh. 14 - Calculate G for the following reactions at 25C....Ch. 14 - Calculate G for the following reactions at 25C....Ch. 14 - From the values of H and S, predict which of the...Ch. 14 - Find the temperatures at which reactions with the...Ch. 14 - The molar heats of fusion and vaporization of...Ch. 14 - The molar heats of fusion and vaporization of...Ch. 14 - Use the values listed in Appendix 2 to calculate G...Ch. 14 - Certain bacteria in the soil obtain the necessary...Ch. 14 - What is a coupled reaction? What is its importance...Ch. 14 - What is the role of ATP in biological reactions?Ch. 14 - Prob. 14.40QPCh. 14 - Predict the signs of H, S, and G of the system for...Ch. 14 - A student placed 1 g of each of three compounds A,...Ch. 14 - The enthalpy change in the denaturation of a...Ch. 14 - Consider the following facts: Water freezes...Ch. 14 - Ammonium nitrate (NH4NO3) dissolves spontaneously...Ch. 14 - The standard enthalpy of formation and the...Ch. 14 - (a) Troutons rule states that the ratio of the...Ch. 14 - Referring to Problem 14.47, explain why the ratio...Ch. 14 - Prob. 14.49QPCh. 14 - Prob. 14.50QPCh. 14 - Prob. 14.51QPCh. 14 - Prob. 14.52QPCh. 14 - Prob. 14.53QPCh. 14 - The molar heat of vaporization of ethanol is 39 3...Ch. 14 - As an approximation, we can assume that proteins...Ch. 14 - When a native protein in solution is heated to a...Ch. 14 - A 74.6-g ice cube floats in the Arctic Sea. The...Ch. 14 - A reaction for which H and S are both negative is...Ch. 14 - The sublimation of carbon dioxide at 78C is given...Ch. 14 - Many hydrocarbons exist as structural isomers,...Ch. 14 - Consider the following reaction at 298 K. 2H2(s) +...Ch. 14 - Which of the following is not accompanied by an...Ch. 14 - Which of the following are not state functions: S,...Ch. 14 - Give a detailed example of each of the following,...Ch. 14 - Hydrogenation reactions (e.g., the process of...Ch. 14 - At 0 K. the entropy of carbon monoxide crystal is...Ch. 14 - Which of the following thermodynamic functions are...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why the representation of a one-dimensional velocity distribution function for a particular gas becomes flatter as the temperature increases.arrow_forwardDraw a Lewis structure for each of the following molecules and assign charges where appropriate. The order in which the atoms are connected is given in parentheses. a. CIFCIF b. BrCNBrCN 0 c. SOCI2 × (CISCIO) SOC₁₂ (CISCI) You can draw both an octet and a valence shell expanded structure. Considering the following structural information, which is the better one: The measured S-OS-O bond length in SOC12SOCl2 is 1.43 Å. For comparison, that in SO2SO2 is 1.43 Å [Exercise 1-9, part (b)], that in CHзSOHCH3 SOH d. CH3NH2CH3NH2 (methanesulfenic acid) is 1.66 A. e. CH3OCH3 CH3 OCH3 NH2 f. N2H2× (HNNH) N2 H2 (HNNH) g. CH2COCH₂ CO h. HN3× (HNNN) HN3 (HNNN) i. N20 × (NNO) N2O (NNO)arrow_forwardbre The reaction sequence shown in Scheme 5 demonstrates the synthesis of a substituted benzene derivative Q. wolsd works 2 NH2 NaNO2, HCI (apexe) 13× (1 HNO3, H2SO4 C6H5CIN2 0°C HOTE CHINO₂ N O *O₂H ( PO Q Я Scheme 5 2 bag abouoqmics to sounde odi WEIC (i) Draw the structure of intermediate O. [2 marks] to noitsmot od: tot meinedogm, noit so oft listsb ni zaupaib bas wa (ii) Draw the mechanism for the transformation of aniline N to intermediate O. Spoilage (b) [6 marks] (iii) Identify the reagent X used to convert compound O to the iodinated compound [tom E P. vueimado oilovonsa ni moitos nolisbnolov ayd toes ai tedw nisiqx (iv) Identify the possible structures of compound Q. [2 marks] [2 marks] [shom 2] (v) bus noires goiribbeolovo xnivollot adj to subora sidab Draw the mechanism for the transformation of intermediate P to compound Q. [5 marks] vi (vi) Account for the regiochemical outcome observed in the reaction forming compound Q. [3 marks]arrow_forward
- Please correct answer and don't used hand raitingarrow_forwardThe vibrational contribution isa) temperature independent for internal energy and heat capacityb) temperature dependent for internal energy and heat capacityc) temperature independent for heat capacityd) temperature independent for internal energyarrow_forwardQuantum mechanics. Explain the basis of approximating the summation to an integral in translational motion.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY