
Concept explainers
Write structural formulas for the following compounds.
- a. ethyl isopropyl ether
- b. di-n-butyl ether
- c. 2-ethoxyoctane
- d. divinyl ether
- e. allyl methyl ether
- f. cyclohexene oxide
- g. cis-2,3-epoxyhexane
- h. (2R, 3S)-2-methoxypentan-3-ol
- i. trans-2,3-dimethyloxirane
(a)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of ethyl isopropyl ether is shown in Figure 1.
Explanation of Solution
The given compound is ethyl isopropyl ether. The root name isopropyl signifies three carbon atoms in a chain. Ethyl and isopropyl are the groups attached to an oxygen atom. The structure of ethyl isopropyl ether is shown below.
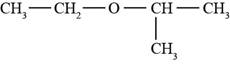
Figure 1
(b)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of di-n-butyl ether is shown in Figure 2.
Explanation of Solution
The given compound is di-n-butyl ether. The root name butyl signifies four carbon atoms in a chain and di-n-butyl indicates that two butyl groups are attached to an oxygen atom. The structure of di-n-butyl ether is shown below.
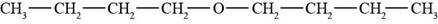
Figure 2
(c)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of 2-ethoxyoctane is shown in Figure 3.
Explanation of Solution
The given compound is 2-ethoxyoctane. The root octane signifies eight carbon atoms in a chain and 2-ethoxy shows that ethoxy group is present at second carbon atom of a chain. The structure of 2-ethoxyoctane is shown below.
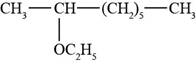
Figure 3
(d)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of divinyl ether is shown in Figure 4.
Explanation of Solution
The given compound is divinyl ether. The Vinyl group is the functional group having a formula
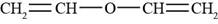
Figure 4
(e)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of allyl methyl ether is shown in Figure 5.
Explanation of Solution
The given compound is allyl methyl ether. An allyl group is used as a substituent and has a formula of
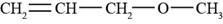
Figure 5
(f)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of cyclohexene oxide is shown in Figure 6.
Explanation of Solution
The given compound is cyclohexene oxide. The root name cyclohexene signifies six carbon atoms in a ring and oxide indicated the presence of epoxide ring. The structure of cyclohexene oxide is shown below.
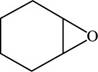
Figure 6
(g)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of
Explanation of Solution
The given compound is
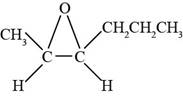
Figure 7
(h)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of
Explanation of Solution
The given compound is
According to Fisher projection,
For
The structure of
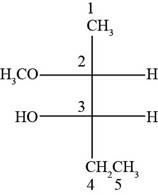
Figure 8
(i)
Interpretation:
The structure formula for the given compound is to be drawn.
Concept introduction:
An organic compound where an oxygen atom is attached to two alkyl or aryl groups is known as ether. The general formula of ethers is
Answer to Problem 14.29SP
The structure of
Explanation of Solution
The given compound is
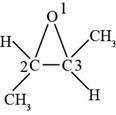
Figure 9
Want to see more full solutions like this?
Chapter 14 Solutions
ORGANIC CHEMISTRY
- EFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardIf we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardYou need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardComplete the following reactions- hand written pleasearrow_forwardGive the organic product: O A O B Ос ○ D -NH–CH3 + CH3 CH3 NEN C ? A CH3 CH3 NH- CH3 B CH3 CH3 N=N- C CH3 CH3 N=NNH CH3 D CH3 N=N CH3 NHCH3 LNH CHOarrow_forward
- Finish the reaction- hand written pleasearrow_forwardGive the organic products: (benzyne) Br ? CH3 + K* :NH, liq NH3 HINT: Two products are formed. Each is a substituted aniline; they are isomers of each other. NH2 II I H₂N. CH3 CH3 III Select one: ○ A. I and II ○ B. I and III O C. I and IV O D. II and III O E. III and IV H₂N CH3 IV CH₂-NH2arrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Erase something Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





