
Concept explainers
(a)
Interpretation:
The product on reaction of
Concept introduction:
The
Answer to Problem 14.26AP
The product on reaction of
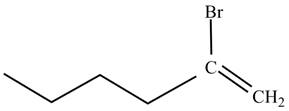
Explanation of Solution
The reaction of
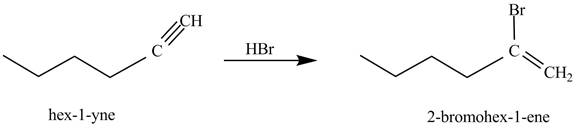
Figure 1
In the above reaction, hexyne reacts with ![]() to form a haloalkene. Therefore, the product on reaction of
to form a haloalkene. Therefore, the product on reaction of
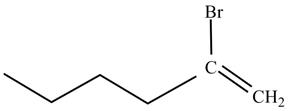
Figure 2
The product on reaction of
(b)
Interpretation:
The product on reaction of
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The product on reaction of
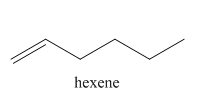
Explanation of Solution
The reaction of
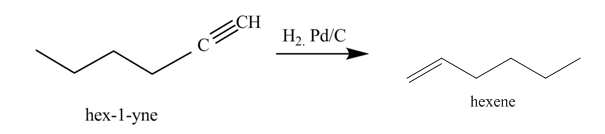
Figure 3
In the above reaction, hexyne reacts with hydrogen to form hexene. Hexyne is an unsaturated molecule consisting of a triple bond. It reacts hydrogen in presence of

Figure 4
The product on reaction of
(c)
Interpretation:
The product on reaction of
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The product on reaction of
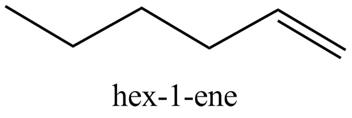
Explanation of Solution
The reaction of
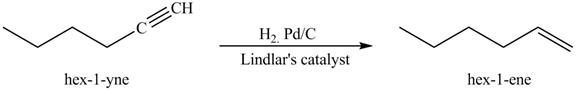
Figure 5
In the above reaction, hexyne reacts with
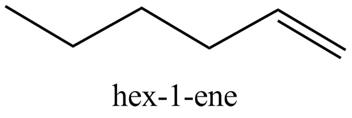
Figure 6
The product on reaction of
(d)
Interpretation:
The product on reaction of the product formed in part (c) with
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The product on reaction of the product formed in part (c) with
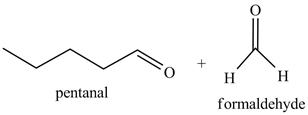
Explanation of Solution
The product formed in part (c) is shown below.
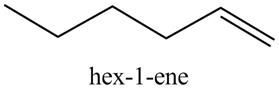
Figure 7
The reaction of the product formed in part (c) with
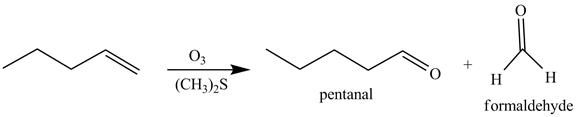
Figure 8
The above reaction is known as ozonlysis reaction. In the above reaction, a double bond is cleaved and oxidized to give two products. Reaction of hexene with

Figure 9
The products on reaction of reaction of the product formed in part (c) with
(e)
Interpretation:
The product on reaction of the product formed in part (c) with
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The product on reaction of the product formed in part (c) with
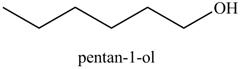
Explanation of Solution
The product formed in part (c) is shown below.

Figure 7
The reaction of above compound with
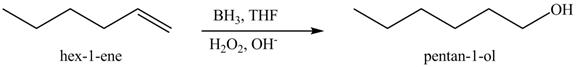
Figure 10
In the above reaction, hexene reacts with boron hydride to form organoborane which further reacts with peroxide to form an alcohol. The alcohol thus formed is by anti-markovnikov addition.

Figure 11
The product on reaction of the product formed in part (c) with
(f)
Interpretation:
The product on reaction of the product formed in part (c) with
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The product on reaction of the product formed in part (c) with
Explanation of Solution
The product formed in part (c) is shown below.
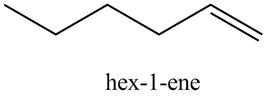
Figure 7
The reaction of above compound with
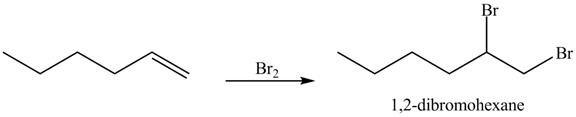
Figure 12
In the above reaction, hexene being unsaturated reacts with bromine molecule to form a dibromoalkane, that is
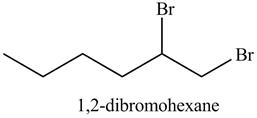
Figure 13
The product on reaction of the product formed in part (c) with
(g)
Interpretation:
The product on reaction of
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The product on reaction of
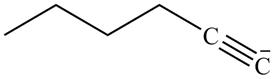
Explanation of Solution
The reaction of
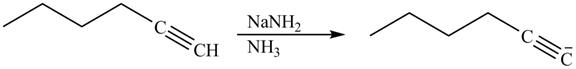
Figure 14
Alkynes react with sodamide

Figure 15
The product on reaction of
(h)
Interpretation:
The product on reaction of the product formed in part (g) with
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The product on reaction of the product formed in part (g) with
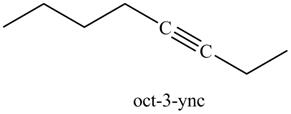
Explanation of Solution
The product formed in part (g) is shown below.
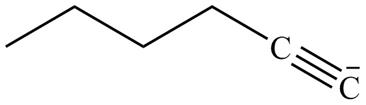
Figure 15
The reaction of above compound with
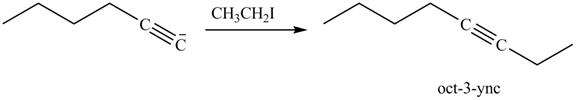
Figure 16
In the above reaction, the anion of alkyne obtained reacts with iodoethane to form an alkyne of eight carbons. It is non-terminal alkyne, that is, triple bond is not situated at the terminal end. The product formed is shown below.
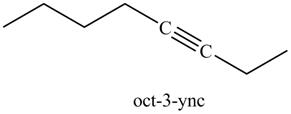
Figure 17
The product on reaction of the product formed in part (g) with
(i)
Interpretation:
The product on reaction of
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The product on reaction of
Explanation of Solution
The reaction of
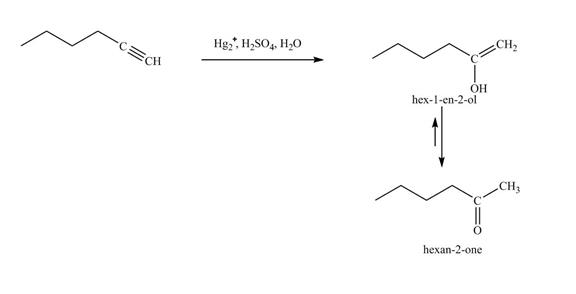
Figure 18
In the above reaction, hexyne reacts with

Figure 19
The product on reaction of
(j)
Interpretation:
The product on reaction of
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The product on reaction of
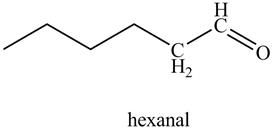
Explanation of Solution
The reaction of
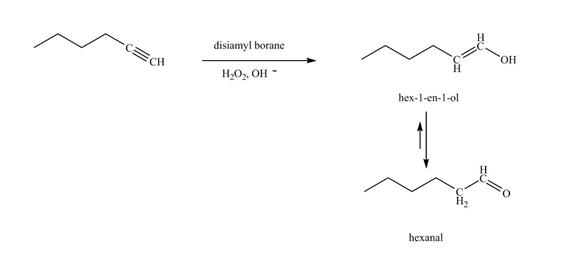
Figure 20
In the above reaction, hexyne reacts with disiamyl borane to form an organoborane. This reaction is also known as hydroboration-oxidation reaction. The organo-borane gives an enol which tautomerism to form an
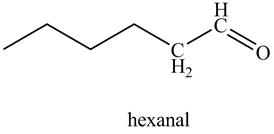
Figure 21
The product on reaction of
(k)
Interpretation:
The product on reaction of
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The products formed on reaction of
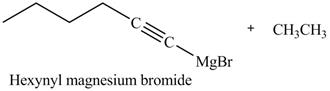
Explanation of Solution
The reaction of
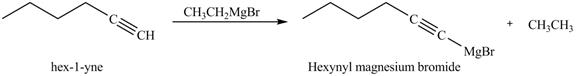
Figure 22
In the above reaction,

Figure 23
The products formed on reaction of
(l)
Interpretation:
The product on reaction of the product formed in part (k) with ethylene oxide and
Concept introduction:
The alkynes consist of a triple bond between two carbon atoms. The general formula of alkynes is
Answer to Problem 14.26AP
The product on reaction of the product formed in part (k) with ethylene oxide and
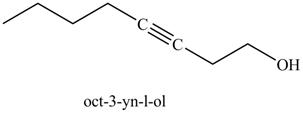
Explanation of Solution
The product formed in part (k) is shown below.
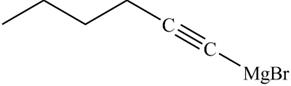
Figure 23
The reaction of above compound with ethylene oxide and
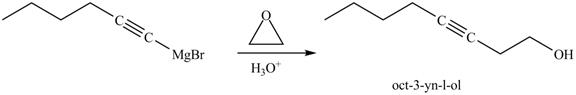
Figure 24
In the above reaction, the Grignard reagent obtained in part (k) reacts with ethylene oxide followed by hydrolysis to form an alcohol with a triple bond in between the chain. The product formed on reaction of product formed in part (k) with ethylene oxide and
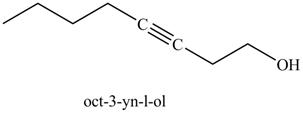
Figure 25
The product on reaction of the product formed in part (k) with ethylene oxide and
Want to see more full solutions like this?
Chapter 14 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





