
MAST F/ CHEM: THE CENTRAL SCI CODE ALON
14th Edition
ISBN: 9781323654378
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 11E
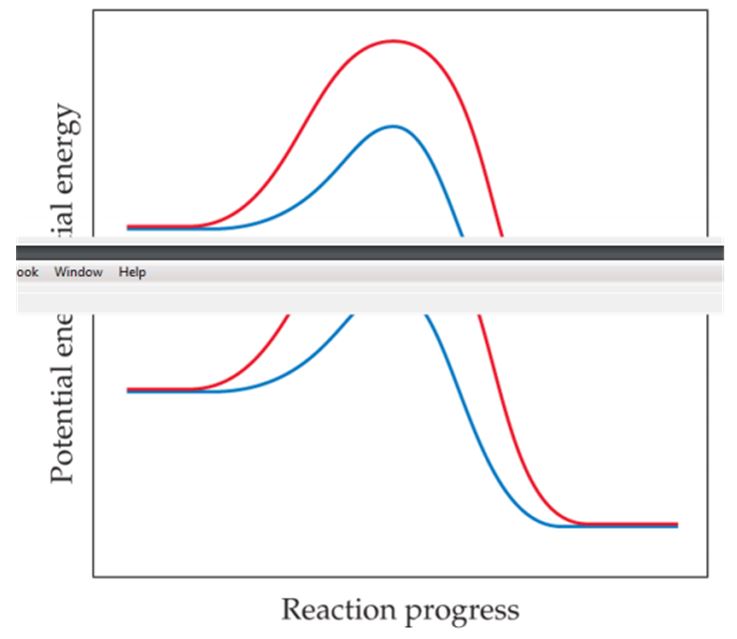
The following graph shows two different reaction pathways for the same overall reaction at the same temperature. Is each of the following statements true or false? (a) The rate is faster for the red path than for the blue path. (b) For both paths, the rate of the reverse reaction is slower than the rate of the forward reaction. (c) The energy change ΔE is the same for both paths. [Section 14.6]
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
Dr. Mendel asked his BIOL 260 class what their height was and what their
parent's heights were. He plotted that data in the graph below to determine if
height was a heritable trait.
A. Is height a heritable trait? If yes, what is the heritability value? (2 pts)
B. If the phenotypic variation is 30, what is the variation due to additive alleles?
(2 pts)
Offspring Height (Inches)
75
67.5
60
52.5
y = 0.9264x + 4.8519
55
60
65
MidParent Height (Inches)
70
75
12pt v
V
Paragraph B IUA
>
AT2 v
V
Experiment:
Each team will be provided with 5g of a mixture of acetanilide and salicylic acid. You will divide it into three 1.5 g portions in separate 125 mL Erlenmeyer flasks savıng some for melting point analysis. Dissolve the mixture in each flask in ~60mL of DI water by heating to boiling on a hotplate. Take the flasks off the hotplate once you have a clear solution and let them stand on the bench top for 5 mins and then allow them to cool as described below.
Sample A-Let the first sample cool slowly to room temperature by letting it stand on your lab bench, with occasional stirring to promote crystallization.
Sample B-Cool the second sample 1n a tap-water bath to 10-15 °C
Sample C-Cool the third sample in an ice-bath to 0-2 °C
Results:
weight after recrystalization and melting point temp.
A=0.624g,102-115°
B=0.765g, 80-105°
C=1.135g, 77-108
What is the percent yield of A,B, and C.
Chapter 14 Solutions
MAST F/ CHEM: THE CENTRAL SCI CODE ALON
Ch. 14.2 - If the experiment in Figure 14.2 is run for 60 s,...Ch. 14.2 - Prob. 14.1.2PECh. 14.2 - Which of the following could be the instantaneous...Ch. 14.2 - Using Figure 14.3, determine the instantaneous...Ch. 14.2 - At a certain time in a reaction, substance A is...Ch. 14.2 - Prob. 14.3.2PECh. 14.3 - Suppose the rate law for the reaction in this...Ch. 14.3 - Assuming that rate = k[A][B], rank the mixtures...Ch. 14.3 - Prob. 14.5.1PECh. 14.3 - Prob. 14.5.2PE
Ch. 14.3 - Consider the reaction examined above in the Sample...Ch. 14.3 - The following data were measured for the reaction...Ch. 14.4 - At 25 ° C, the decomposition of dinitrogen...Ch. 14.4 - Practice Exercise 2 The decomposition of dimethyl...Ch. 14.4 - Practice Exercise 1 For a certain reaction A ...Ch. 14.4 - Prob. 14.8.2PECh. 14.4 - Practice Exercise 1 We noted in an earlier...Ch. 14.4 - Practice Exercise 2 Using Equation 14.17,...Ch. 14.5 - Practice Exercise 1 This of the following change...Ch. 14.5 - Practice Exercise 2 Rank the rate constants of the...Ch. 14.5 - Practice Exercise 1 Using the data in Sample...Ch. 14.5 - Practice Exercise 2 To one significant figure,...Ch. 14.6 - Practice Exercise 1 Consider the following...Ch. 14.6 - For the reaction Mo(CO)6 +P(CH3)3 Mo(CO)5P(CH3)3...Ch. 14.6 - Practice Exercise 1 Consider the following...Ch. 14.6 - Practice Exercise 2 Consider the following...Ch. 14.6 - Practice Exercise 1 An Alternative two-step...Ch. 14.6 - Prob. 14.14.2PECh. 14.6 - Practice Exercise 1
Consider the...Ch. 14.6 - Prob. 14.15.2PECh. 14 - Prob. 1DECh. 14 - An automotive fuel injector dispenses a fine spray...Ch. 14 - Consider the following graph of the concentration...Ch. 14 - You study the rate of a reaction, measuring both...Ch. 14 - Suppose that for the reaction K+L M, you monitor...Ch. 14 - Prob. 5ECh. 14 - A friend studies a first-order reaction and...Ch. 14 - Prob. 7ECh. 14 - Which of the following linear plots do you expect...Ch. 14 - Prob. 9ECh. 14 - Prob. 10ECh. 14 - The following graph shows two different reaction...Ch. 14 - Prob. 12ECh. 14 - Prob. 13ECh. 14 - Draw a possible transition state for the...Ch. 14 - The following diagram represents an imaginary...Ch. 14 - 14.16 Draw a graph showing the reaction pathway...Ch. 14 - Prob. 17ECh. 14 - 14.18 (a) what are the units usually used to...Ch. 14 - Prob. 19ECh. 14 - A flask is charged with 0.100 mol of A and allowed...Ch. 14 - The isomerization of methyl isontrile (CH3NC) to...Ch. 14 - Prob. 22ECh. 14 - Prob. 23ECh. 14 - For each of the following gas-phase reactions,...Ch. 14 - (a) Consider the combustion of hydrogen, 2H2 (g) +...Ch. 14 - Prob. 26ECh. 14 - A reaction A+B C obeys the following rate law:...Ch. 14 - Prob. 28ECh. 14 - 14.29 The decomposition reaction of N2O5 in carbon...Ch. 14 - Prob. 30ECh. 14 - Prob. 31ECh. 14 - The reaction between ethyl bromide (C2H5Br) and...Ch. 14 - Prob. 33ECh. 14 - The reaction 2ClO2 (aq) + 2OH- (aq) ClO3- (aq) +...Ch. 14 - The following data were measured for the reaction...Ch. 14 - The following data were collected for the rate of...Ch. 14 - Consider the gas-phase reaction between nitric...Ch. 14 - Prob. 38ECh. 14 - Prob. 39ECh. 14 - Prob. 40ECh. 14 - Prob. 41ECh. 14 - Molecular iodine, I2 (g), dissociates into iodine...Ch. 14 - Prob. 43ECh. 14 - Prob. 44ECh. 14 - The reaction SO2Cl2 (g) O2 (g) + Cl2 (g) is first...Ch. 14 - Prob. 46ECh. 14 - Prob. 47ECh. 14 - Prob. 48ECh. 14 - Prob. 49ECh. 14 - Prob. 50ECh. 14 - (a) what factors determine whether a collision...Ch. 14 - (a) in which of the following reactions you expect...Ch. 14 - Calculate the fraction of atoms in a sample of...Ch. 14 - (a) the activation energy for the isomerization of...Ch. 14 - The gas-phase reaction CL (g) + HBr (g) + HCl (g)...Ch. 14 - Prob. 56ECh. 14 - Indicate whether each statement is true or false....Ch. 14 - Indicate whether each statement is true or false....Ch. 14 - Based on their activation energies and energy...Ch. 14 - Prob. 60ECh. 14 - Prob. 61ECh. 14 - Prob. 62ECh. 14 - The rate of the reaction CH3COOC2H5 (aq) + OH- ...Ch. 14 - Prob. 64ECh. 14 - Prob. 65ECh. 14 - Prob. 66ECh. 14 - What is the molecularity of each of the following...Ch. 14 - Prob. 68ECh. 14 - (a) based on the following reaction profile, how...Ch. 14 - Prob. 70ECh. 14 - Prob. 71ECh. 14 - Prob. 72ECh. 14 - The reaction 2NO (g) + CL2 (g) 2NOCl (g) was...Ch. 14 - You have studied the gas-phase oxidation of HBr by...Ch. 14 - Prob. 75ECh. 14 - Prob. 76ECh. 14 - Prob. 77ECh. 14 - Prob. 78ECh. 14 - Prob. 79ECh. 14 - The addition of No accelerates the decomposition...Ch. 14 - 14.81b Many metallic catalysts, particularly the...Ch. 14 - Prob. 82ECh. 14 - When D2 reacts with ethylene (C2H4) in the...Ch. 14 - Prob. 84ECh. 14 - Prob. 85ECh. 14 - The enzyme urease catalyzez the reaction of urea,(...Ch. 14 - Prob. 87ECh. 14 - Prob. 88ECh. 14 - Prob. 89AECh. 14 - Prob. 90AECh. 14 - Prob. 91AECh. 14 - Prob. 92AECh. 14 - Prob. 93AECh. 14 - Prob. 94AECh. 14 - Prob. 95AECh. 14 - Prob. 96AECh. 14 - [14.97]A first order reaction A B has the rate...Ch. 14 - Prob. 98AECh. 14 - Prob. 99AECh. 14 - Prob. 100AECh. 14 - Prob. 101AECh. 14 - Prob. 102AECh. 14 - Cyclopentadiene (C5H6) reacts with itself to form...Ch. 14 - Prob. 104AECh. 14 - At 280C, raw milk sours in 4.0 h but takes 48 h to...Ch. 14 - Prob. 106AECh. 14 - Prob. 107AECh. 14 - Prob. 108AECh. 14 - Prob. 109AECh. 14 - The following mechanism has been proposed for the...Ch. 14 - Prob. 111AECh. 14 - Prob. 112AECh. 14 - Platinum nanoparticles of diameter ~2 nm are...Ch. 14 - 14.114 One of the many remarkable enzymes in the...Ch. 14 - 14.115N Suppose that, in the absence of catalyst,...Ch. 14 - Prob. 116AECh. 14 - Dinitrogen pentoxide (N2O5) decomposes in...Ch. 14 - The reaction between ethyl iodide and hydroxide...Ch. 14 - Prob. 119IECh. 14 - Prob. 120IECh. 14 - Prob. 121IECh. 14 - The rates of many atmospheric reactions are...Ch. 14 - Prob. 123IECh. 14 - Prob. 124IECh. 14 - Prob. 125IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rel. Intensity Q 1. Which one of the following is true of the compound whose mass spectrum is shown here? Explain how you decided. 100 a) It contains chlorine. b) It contains bromine. c) It contains neither chlorine nor bromine. 80- 60- 40- 20- 0.0 0.0 TT 40 80 120 160 m/z 2. Using the Table of IR Absorptions how could you distinguish between these two compounds in the IR? What absorbance would one compound have that the other compound does not? HO CIarrow_forwardIllustrate reaction mechanisms of alkenes with water in the presence of H2SO4, detailing each step of the process. Please show steps of processing. Please do both, I will thumb up for sure #1 #3arrow_forwardDraw the following molecule: (Z)-1-chloro-1-butenearrow_forward
- Identify the molecule as having a(n) E, Z, cis, or trans configuration. CH3 H₁₂C ○ E ○ z ○ cis transarrow_forwardIdentify the molecule as having a(n) E, Z, cis, or trans configuration. H₂C- CH3 О Е ○ cis ○ transarrow_forwardThe decomposition of dinitrogen pentoxide according to the equation: 50°C 2 N2O5(g) 4 NO2(g) + O2(g) follows first-order kinetics with a rate constant of 0.0065 s-1. If the initial concentration of N2O5 is 0.275 M, determine: the final concentration of N2O5 after 180 seconds. ...arrow_forward
- Don't used hand raitingarrow_forwardCS2(g) →CS(g) + S(g) The rate law is Rate = k[CS2] where k = 1.6 × 10−6 s−¹. S What is the concentration of CS2 after 5 hours if the initial concentration is 0.25 M?arrow_forwardCS2(g) → CS(g) + S(g) The rate law is Rate = k [CS2] where k = 1.6 × 10-6 s−1. S Calculate the half-life.arrow_forward
- The following is a first order reaction where the rate constant, k, is 6.29 x 10-3 min-*** What is the half-life? C2H4 C2H2 + H2arrow_forwardControl Chart Drawing Assignment The table below provides the number of alignment errors observed during the final inspection of a certain model of airplane. Calculate the central, upper, and lower control limits for the c-chart and draw the chart precisely on the graph sheet provided (based on 3-sigma limits). Your chart should include a line for each of the control limits (UCL, CL, and LCL) and the points for each observation. Number the x-axis 1 through 25 and evenly space the numbering for the y-axis. Connect the points by drawing a line as well. Label each line drawn. Airplane Number Number of alignment errors 201 7 202 6 203 6 204 7 205 4 206 7 207 8 208 12 209 9 210 9 211 8 212 5 213 5 214 9 215 8 216 15 217 6 218 4 219 13 220 7 221 8 222 15 223 6 224 6 225 10arrow_forwardCollagen is used to date artifacts. It has a rate constant = 1.20 x 10-4 /years. What is the half life of collagen?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY