
EBK CHEMISTRY: AN ATOMS FIRST APPROACH
2nd Edition
ISBN: 9780100552234
Author: ZUMDAHL
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 114CP
Consider the following two acids:
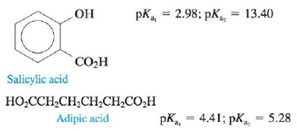
In two separate experiments the pH was measured during the titration of 5.00 mmol of each acid with 0.200 M NaOH. Each experiment showed only one stoichiometric point when the data were plotted. In one experiment the stoichiometric point was at 25.00 mL added NaOH, and in the other experiment the stoichiometric point was at 50.00 mL NaOH. Explain these results. (See Exercise 113.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Name the molecules & Identify any chiral center
CH3CH2CH2CHCH₂CH₂CH₂CH₂
OH
CH₂CHCH2CH3
Br
CH3
CH3CHCH2CHCH2CH3
CH3
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Chapter 14 Solutions
EBK CHEMISTRY: AN ATOMS FIRST APPROACH
Ch. 14 - What is meant by the presence of a common ion? How...Ch. 14 - Define a buffer solution. What makes up a buffer...Ch. 14 - Prob. 3RQCh. 14 - A good buffer generally contains relatively equal...Ch. 14 - Prob. 5RQCh. 14 - Prob. 6RQCh. 14 - Sketch the titration curve for a weak acid...Ch. 14 - Sketch the titration curve for a weak base...Ch. 14 - What is an acidbase indicator? Define the...Ch. 14 - Prob. 10RQ
Ch. 14 - What are the major species in solution after...Ch. 14 - Prob. 2ALQCh. 14 - Prob. 3ALQCh. 14 - Prob. 4ALQCh. 14 - Sketch two pH curves, one for the titration of a...Ch. 14 - Prob. 6ALQCh. 14 - Prob. 7ALQCh. 14 - You have a solution of the weak acid HA and add...Ch. 14 - The common ion effect for weak acids is to...Ch. 14 - Prob. 10QCh. 14 - Prob. 11QCh. 14 - Consider the following pH curves for 100.0 mL of...Ch. 14 - An acid is titrated with NaOH. The following...Ch. 14 - Consider the following four titrations. i. 100.0...Ch. 14 - Prob. 15QCh. 14 - Prob. 16QCh. 14 - How many of the following are buffered solutions?...Ch. 14 - Which of the following can be classified as buffer...Ch. 14 - A certain buffer is made by dissolving NaHCO3 and...Ch. 14 - Prob. 20ECh. 14 - Calculate the pH of each of the following...Ch. 14 - Calculate the pH of each of the following...Ch. 14 - Prob. 23ECh. 14 - Compare the percent ionization of the base in...Ch. 14 - Prob. 25ECh. 14 - Calculate the pH after 0.020 mole of HCl is added...Ch. 14 - Calculate the pH after 0.020 mole of NaOH is added...Ch. 14 - Calculate the pH after 0.020 mole of NaOH is added...Ch. 14 - Which of the solutions in Exercise 21 shows the...Ch. 14 - Prob. 30ECh. 14 - Calculate the pH of a solution that is 1.00 M HNO2...Ch. 14 - Calculate the pH of a solution that is 0.60 M HF...Ch. 14 - Calculate the pH after 0.10 mole of NaOH is added...Ch. 14 - Calculate the pH after 0.10 mole of NaOH is added...Ch. 14 - Calculate the pH of each of the following buffered...Ch. 14 - Prob. 36ECh. 14 - Calculate the pH of a buffered solution prepared...Ch. 14 - A buffered solution is made by adding 50.0 g NH4Cl...Ch. 14 - Prob. 39ECh. 14 - An aqueous solution contains dissolved C6H5NH3Cl...Ch. 14 - Prob. 41ECh. 14 - Prob. 42ECh. 14 - Consider a solution that contains both C5H5N and...Ch. 14 - Calculate the ratio [NH3]/[NH4+] in...Ch. 14 - Prob. 45ECh. 14 - Prob. 46ECh. 14 - Prob. 47ECh. 14 - Prob. 48ECh. 14 - Calculate the pH of a solution that is 0.40 M...Ch. 14 - Calculate the pH of a solution that is 0.20 M HOCl...Ch. 14 - Which of the following mixtures would result in...Ch. 14 - Prob. 52ECh. 14 - Prob. 53ECh. 14 - Calculate the number of moles of HCl(g) that must...Ch. 14 - Consider the titration of a generic weak acid HA...Ch. 14 - Sketch the titration curve for the titration of a...Ch. 14 - Consider the titration of 40.0 mL of 0.200 M HClO4...Ch. 14 - Consider the titration of 80.0 mL of 0.100 M...Ch. 14 - Consider the titration of 100.0 mL of 0.200 M...Ch. 14 - Prob. 60ECh. 14 - Lactic acid is a common by-product of cellular...Ch. 14 - Repeat the procedure in Exercise 61, but for the...Ch. 14 - Repeat the procedure in Exercise 61, but for the...Ch. 14 - Repeat the procedure in Exercise 61, but for the...Ch. 14 - Prob. 65ECh. 14 - In the titration of 50.0 mL of 1.0 M methylamine,...Ch. 14 - You have 75.0 mL of 0.10 M HA. After adding 30.0...Ch. 14 - A student dissolves 0.0100 mole of an unknown weak...Ch. 14 - Prob. 69ECh. 14 - Prob. 70ECh. 14 - Potassium hydrogen phthalate, known as KHP (molar...Ch. 14 - A certain indicator HIn has a pKa of 3.00 and a...Ch. 14 - Prob. 73ECh. 14 - Prob. 74ECh. 14 - Prob. 75ECh. 14 - Prob. 76ECh. 14 - Prob. 77ECh. 14 - Estimate the pH of a solution in which crystal...Ch. 14 - Prob. 79ECh. 14 - Prob. 80ECh. 14 - Prob. 81AECh. 14 - Prob. 82AECh. 14 - Tris(hydroxymethyl)aminomethane, commonly called...Ch. 14 - Prob. 84AECh. 14 - You have the following reagents on hand: Solids...Ch. 14 - Prob. 86AECh. 14 - Prob. 87AECh. 14 - What quantity (moles) of HCl(g) must be added to...Ch. 14 - Calculate the value of the equilibrium constant...Ch. 14 - The following plot shows the pH curves for the...Ch. 14 - Calculate the volume of 1.50 102 M NaOH that must...Ch. 14 - Prob. 92AECh. 14 - A certain acetic acid solution has pH = 2.68....Ch. 14 - A 0.210-g sample of an acid (molar mass = 192...Ch. 14 - The active ingredient in aspirin is...Ch. 14 - One method for determining the purity of aspirin...Ch. 14 - A student intends to titrate a solution of a weak...Ch. 14 - Prob. 98AECh. 14 - Prob. 99AECh. 14 - Consider 1.0 L of a solution that is 0.85 M HOC6H5...Ch. 14 - Prob. 101CWPCh. 14 - Consider the following acids and bases: HCO2H Ka =...Ch. 14 - Prob. 103CWPCh. 14 - Prob. 104CWPCh. 14 - Consider the titration of 100.0 mL of 0.100 M HCN...Ch. 14 - Consider the titration of 100.0 mL of 0.200 M...Ch. 14 - Prob. 107CWPCh. 14 - Prob. 108CPCh. 14 - A buffer is made using 45.0 mL of 0.750 M HC3H5O2...Ch. 14 - A 0.400-M solution of ammonia was titrated with...Ch. 14 - Prob. 111CPCh. 14 - Consider a solution formed by mixing 50.0 mL of...Ch. 14 - When a diprotic acid, H2A, is titrated with NaOH,...Ch. 14 - Consider the following two acids: In two separate...Ch. 14 - The titration of Na2CO3 with HCl bas the following...Ch. 14 - Prob. 116CPCh. 14 - A few drops of each of the indicators shown in the...Ch. 14 - Malonic acid (HO2CCH2CO2H) is a diprotic acid. In...Ch. 14 - A buffer solution is prepared by mixing 75.0 mL of...Ch. 14 - A 10.00-g sample of the ionic compound NaA, where...Ch. 14 - Prob. 121IPCh. 14 - Prob. 122MP
Additional Science Textbook Solutions
Find more solutions based on key concepts
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY