
ORGANIC CHEMISTRY-PRINT COMPANION (LL)
4th Edition
ISBN: 9781119659594
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13.8, Problem 14ATS
Interpretation Introduction
Interpretation: For the given reaction, the structure of compound 2 needs to be drawn. Also, reagents used for converting 1 into 3 need to be identified.
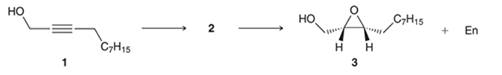
Concept introduction:
Preparation of epoxide-
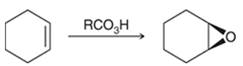
Peroxy acids generally used in this process are MCPBA and peroxyacetic acid. The formation of epoxide via peroxy acid is a stereospecific process; thus, cis substituents in alkene (starting material) remain at cis to each other in the epoxide (product). Similarly, trans substituents in alkene remain at trans to each other.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Protecting Groups and Carbonyls
6) The synthesis generates allethrolone that exhibits high insect toxicity but low mammalian toxicity. They are used in pet
shampoo, human lice shampoo, and industrial sprays for insects and mosquitos. Propose detailed mechanistic steps to
generate the allethrolone label the different types of reagents (Grignard, acid/base protonation, acid/base deprotonation,
reduction, oxidation, witting, aldol condensation, Robinson annulation, etc.)
III + VI
HS
HS
H+
CH,CH,Li
III
I
II
IV
CI + P(Ph)3
V
༼
Hint: no strong base added
VI
S
VII
IX
HO
VIII
-MgBr
HgCl2,HgO
HO.
isomerization
aqeuous solution
H,SO,
༽༽༤༽༽
X
MeOH
Hint: enhances selectivity for reaction at the S
X
☑
Draw the complete mechanism for the acid-catalyzed hydration of this alkene.
esc
田
Explanation
Check
1
888
Q
A
slock
Add/Remove step
Q
F4
F5
F6
A
བྲA
F7
$
%
5
@
4
2
3
&
6
87
Click and drag to start
drawing a structure.
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce
W
E
R
T
Y
U
S
D
LL
G
H
IK
DD
요
F8
F9
F10
F1
*
(
8
9
0
O
P
J
K
L
Z
X
C
V
B
N
M
H
He
command
Explanation
Check
F1
H₂O
H₂
Pd
1) MCPBA
2) H3O+
1) Hg(OAc)2, H₂O
2) NaBH4
OH
CI
OH OH
OH
hydration
halohydrin formation
addition
halogenation
hydrogenation
inhalation
hydrogenation
hydration
☐ halohydrin formation
addition
halogenation
formation chelation
hydrogenation
halohydrin formation
substitution
hydration
halogenation
addition
Ohalohydrin formation
subtraction
halogenation
addition
hydrogenation
hydration
F2
80
F3
σ
F4
F5
F6
1
!
2
#
3
$
4
%
05
Q
W
&
Å
© 2025 McGraw Hill LLC. All Rights Reserved.
F7
F8
(
6
7
8
9
LU
E
R
T
Y
U
A
F9
Chapter 13 Solutions
ORGANIC CHEMISTRY-PRINT COMPANION (LL)
Ch. 13.2 - Prob. 1LTSCh. 13.2 - Prob. 1PTSCh. 13.2 - Prob. 2PTSCh. 13.2 - Prob. 3ATSCh. 13.4 - Prob. 4CCCh. 13.5 - Prob. 2LTSCh. 13.5 - Prob. 5PTSCh. 13.5 - Prob. 6ATSCh. 13.5 - Prob. 7CCCh. 13.5 - Prob. 8CC
Ch. 13.5 - Prob. 9CCCh. 13.6 - Prob. 10CCCh. 13.7 - Prob. 11CCCh. 13.7 - Prob. 12CCCh. 13.8 - Prob. 3LTSCh. 13.8 - Prob. 13PTSCh. 13.8 - Prob. 14ATSCh. 13.9 - Prob. 15CCCh. 13.10 - Prob. 4LTSCh. 13.10 - Prob. 17ATSCh. 13.10 - Prob. 5LTSCh. 13.10 - Prob. 19ATSCh. 13.11 - Prob. 20CCCh. 13.12 - Prob. 6LTSCh. 13.12 - Prob. 7LTSCh. 13 - Prob. 26PPCh. 13 - Prob. 27PPCh. 13 - Prob. 28PPCh. 13 - Prob. 29PPCh. 13 - Prob. 30PPCh. 13 - Prob. 31PPCh. 13 - Prob. 32PPCh. 13 - Prob. 33PPCh. 13 - Prob. 34PPCh. 13 - Prob. 35PPCh. 13 - Prob. 36PPCh. 13 - Prob. 37PPCh. 13 - Prob. 38PPCh. 13 - Prob. 39PPCh. 13 - Prob. 40PPCh. 13 - Prob. 41PPCh. 13 - Prob. 42PPCh. 13 - Prob. 43PPCh. 13 - Prob. 44PPCh. 13 - Prob. 45PPCh. 13 - Prob. 46ASPCh. 13 - Prob. 47ASPCh. 13 - Prob. 48ASPCh. 13 - Prob. 49ASPCh. 13 - Prob. 50ASPCh. 13 - Prob. 51ASPCh. 13 - Prob. 52ASPCh. 13 - Prob. 53ASPCh. 13 - Prob. 54IPCh. 13 - Prob. 59IPCh. 13 - Prob. 60IPCh. 13 - Prob. 61IPCh. 13 - Prob. 62IPCh. 13 - Prob. 63IPCh. 13 - Prob. 64IPCh. 13 - Prob. 65IPCh. 13 - Prob. 66IPCh. 13 - Prob. 69IPCh. 13 - Prob. 70IPCh. 13 - Prob. 71IPCh. 13 - Prob. 72IPCh. 13 - Prob. 73IPCh. 13 - Prob. 74IPCh. 13 - Prob. 77CPCh. 13 - Prob. 79CPCh. 13 - Prob. 80CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- The two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forwardасть Identify all the bonds that gauche interact with C-OMe in the most stable conformation of the above compound.arrow_forwardPredict the reactants used in the formation of the following compounds using Acid-Catalyzed dehydration reactionarrow_forward
- Can I please get help with this?arrow_forward.. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show ll relevant stereochemistry [3 ONLY]. A H Br 1. NaCN 2 NaOH, H₂O, heat 3. H3O+ B. CH₂COOH 19000 1. LiAlH4 THF, heat 2 H₂O* C. CH Br 1. NaCN, acetone 2 H3O+, heat D. Br 1. Mg. ether 3. H₂O+ 2 CO₂ E. CN 1. (CH) CHMgBr, ether 2 H₂O+arrow_forwardAssign this COSY spectrumarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License