
Concept explainers
(a)
Interpretation: The major product formed in the given reaction needs to be identified.
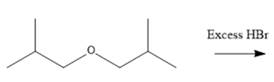
Concept Introduction: To solve the given problem, reactions shown by ethers need to be studied in detail. Generally, ether does not react under mildly acidic or basic conditions. Because of this, they are used as a solvent in many reactions. Ethers can show an acidic cleavage reaction when heated in the presence of excess HX. This process involves two substitution reactions.
(b)
Interpretation: The major product formed in the given reaction needs to be identified.
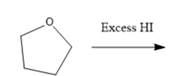
Concept Introduction: To solve the given problem, reactions shown by ethers need to be studied in detail. Generally, ether does not react under mildly acidic or basic conditions. Because of this, they are used as a solvent in many reactions. Ethers can show an acidic cleavage reaction when heated in the presence of excess HX. This process involves two substitution reactions.
(c)
Interpretation: The major product formed in the given reaction needs to be identified.
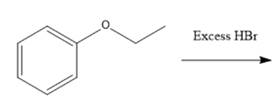
Concept Introduction: To solve the given problem, reactions shown by ethers need to be studied in detail. Generally, ether does not react under mildly acidic or basic conditions. Because of this, they are used as a solvent in many reactions. Ethers can show an acidic cleavage reaction when heated in the presence of excess HX. This process involves two substitution reactions.
(d)
Interpretation: The major product formed in the given reaction needs to be identified.
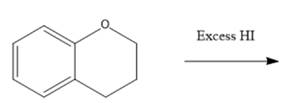
Concept Introduction: To solve the given problem, reactions shown by ethers need to be studied in detail. Generally, ether does not react under mildly acidic or basic conditions. Because of this, they are used as a solvent in many reactions. Ethers can show an acidic cleavage reaction when heated in the presence of excess HX. This process involves two substitution reactions.
(e)
Interpretation: The major product formed in the given reaction needs to be identified.
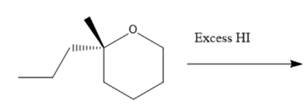
Concept Introduction: To solve the given problem, reactions shown by ethers need to be studied in detail. Generally, ether does not react under mildly acidic or basic conditions. Because of this, they are used as a solvent in many reactions. Ethers can show an acidic cleavage reaction when heated in the presence of excess HX. This process involves two substitution reactions.
(f)
Interpretation: The major product formed in the given reaction needs to be identified.
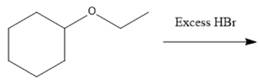
Concept Introduction: To solve the given problem, reactions shown by ethers need to be studied in detail. Generally, ether does not react under mildly acidic or basic conditions. Because of this, they are used as a solvent in many reactions. Ethers can show an acidic cleavage reaction when heated in the presence of excess HX. This process involves two substitution reactions.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Indicate the substitutes in one place, if they are a diazonio room.arrow_forwardIndicate the product formed in each reaction. If the product exhibits tautomerism, draw the tautomeric structure. a) о + CH3-NH-NH2 CO2C2H5 b) + CoH5-NH-NH2 OC2H5arrow_forwardIndicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forward
- Synthesis of 1-metilbenzotriazole from 1,2-diaminobenceno.arrow_forwardSynthesis of 1-metilbenzotriazole.arrow_forwardIndicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forward
- Identify the mechanism through which the following reaction will proceed and draw the major product. Part 1 of 2 Br KOH EtOH Through which mechanism will the reaction proceed? Select the single best answer. E1 E2 neither Part: 1/2 Part 2 of 2 Draw the major product formed as a result of the reaction. Click and drag to start drawing a structure. Xarrow_forwardWhat is single-point calibration? Provide an example.arrow_forwardDraw the major product formed via an E1 pathway.arrow_forward
- Part 9 of 9 Consider the products for the reaction. Identify the major and minor products. HO Cl The E stereoisomer is the major product and the Z stereoisomer is the minor product ▼ S major product minor productarrow_forwardConsider the reactants below. Answer the following questions about the reaction mechanism and products. HO Clarrow_forwardjulietteyep@gmail.com X YSCU Grades for Juliette L Turner: Orc X 199 A ALEKS - Juliette Turner - Modul X A ALEKS - Juliette Turner - Modul x G butane newman projection - Gox + www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBxzaplnN4HsoQggFsejpgqKoyrQrB2dKVAN-BcZvcye0LYa6eXZ8d4vVr8Nc1GZqko5mtw-d1MkNcNzzwZsLf2Tu9_V817y?10Bw7QYjlb il Scribbr citation APA SCU email Student Portal | Main Ryker-Learning WCU-PHARM D MySCU YSCU Canvas- SCU Module 4: Homework (Ch 9-10) Question 28 of 30 (1 point) | Question Attempt: 1 of Unlimited H₂SO heat OH The mechanism of this reaction involves two carbocation intermediates, A and B. Part 1 of 2 KHSO 4 rearrangement A heat B H₂O 2 OH Draw the structure of A. Check Search #t m Save For Later Juliet Submit Assignm 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

