
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 13.5, Problem 13.3CIAP
Interpretation Introduction
Interpretation:
The colour absorbed by the Tetrabromofluorescein dye has to be predicted.
Concept Introduction:
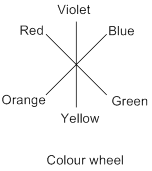
Colour wheel: The arrangement of rainbow colours called ‘VIBGYOR’ around a circle; the interchanging and mixing of primary (red, blue, and yellow) colours between them results in secondary, tertiary colours etc.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
[s] mM
V (M/s)
Uninhibited
0.333
1.65 x 107
1.05 x 107
V (M/s) x 10'
Inhibitor A
V (M/s) x 107
Inhibitor B
0.794 x 107
0.40
1.86 x 107
1.21 x 107
0.893 x 107
0.50
2.13 x 107
1.43 x 107
1.02 x 107
0.666
2.49 x 107
1.74 x 107
1.19 x 107
1.0
2.99 x 107
2.22 x 107
1.43 x 107
2.0
3.72 x 107
3.08 x 107
1.79 x 107
For a Michaelis-Menten reaction, k₁-5 x 10'/M-s, k.-2 x 10%/s, and k₂-4 x 10²/s.
a)
Calculate the Ks and KM for this reaction.
b)
Does substrate binding achieve equilibrium or steady state?
Assume that an enzyme-catalyzed reaction follows the scheme shown:
E+S SES
→E + P
k₁ = 1 x 109/M-s k-1=2.5 x 10%/s k₂ = 3.4 x 107/s
What is the dissociation constant for the enzyme-substrate, K,?
What is the Michaelis constant, Km, for this enzyme?
What is the turnover number, Keat, for this enzyme?
What is the catalytic efficiency for the enzyme?
If the initial Et concentration is 0.25mM, what is Vmax?
Chapter 13 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 13.2 - Prob. 13.1PCh. 13.2 - Prob. 13.2PCh. 13.2 - What are the IUPAC names of the two alkenes shown...Ch. 13.3 - Prob. 13.4PCh. 13.3 - Prob. 13.5PCh. 13.3 - Prob. 13.6KCPCh. 13.5 - Prob. 13.7PCh. 13.5 - Many biological transformations can be simply...Ch. 13.5 - (a) After the reaction of 11-cis-retinal with...Ch. 13.5 - Prob. 13.2CIAP
Ch. 13.5 - Prob. 13.3CIAPCh. 13.6 - Prob. 13.9PCh. 13.6 - Prob. 13.10PCh. 13.6 - Prob. 13.11PCh. 13.6 - Prob. 13.12PCh. 13.6 - Prob. 13.13PCh. 13.6 - Prob. 13.14KCPCh. 13.6 - Prob. 13.1MRPCh. 13.6 - Prob. 13.2MRPCh. 13.6 - Prob. 13.3MRPCh. 13.7 - Prob. 13.15PCh. 13.7 - Prob. 13.16PCh. 13.8 - Prob. 13.17PCh. 13.8 - Polychlorotrifluoroethylene (PCTFE (Kel-F)) is a...Ch. 13.9 - Prob. 13.19PCh. 13.9 - Prob. 13.20PCh. 13.9 - Prob. 13.21KCPCh. 13.10 - What products will be formed when toluene is...Ch. 13.10 - Prob. 13.23PCh. 13.10 - Prob. 13.4CIAPCh. 13.10 - Prob. 13.5CIAPCh. 13.10 - Prob. 13.6CIAPCh. 13 - Prob. 13.24UKCCh. 13 - Prob. 13.25UKCCh. 13 - Prob. 13.26UKCCh. 13 - Draw the product from reaction of the following...Ch. 13 - Prob. 13.28UKCCh. 13 - Prob. 13.29UKCCh. 13 - Prob. 13.30APCh. 13 - Prob. 13.31APCh. 13 - What family-name endings are used for alkenes,...Ch. 13 - Prob. 13.33APCh. 13 - Prob. 13.34APCh. 13 - Write structural formulas for compounds that meet...Ch. 13 - Prob. 13.36APCh. 13 - Prob. 13.37APCh. 13 - Draw structures corresponding to the following...Ch. 13 - Draw structures corresponding to the following...Ch. 13 - Seven alkynes have the formula C6H10. Draw them,...Ch. 13 - Prob. 13.41APCh. 13 - Prob. 13.42APCh. 13 - There are four different pentenes having the...Ch. 13 - Prob. 13.44APCh. 13 - Prob. 13.45APCh. 13 - Draw line structures for the following alkenes....Ch. 13 - Which compound(s) in Problem 13.43 can exist as...Ch. 13 - Prob. 13.48APCh. 13 - Prob. 13.49APCh. 13 - Which of the following pairs are isomers, and...Ch. 13 - Prob. 13.51APCh. 13 - Prob. 13.52APCh. 13 - Prob. 13.53APCh. 13 - Prob. 13.54APCh. 13 - Prob. 13.55APCh. 13 - Prob. 13.56APCh. 13 - Prob. 13.57APCh. 13 - Prob. 13.58APCh. 13 - Prob. 13.59APCh. 13 - What alkene could you use to make the following...Ch. 13 - Prob. 13.61APCh. 13 - Prob. 13.62APCh. 13 - Prob. 13.63APCh. 13 - Prob. 13.64APCh. 13 - For each of the following reagents, decide whether...Ch. 13 - Prob. 13.66APCh. 13 - Prob. 13.67APCh. 13 - Prob. 13.68APCh. 13 - Salicylic acid (o-hydroxybenzoic acid) is used as...Ch. 13 - The following names are incorrect by IUPAC rules....Ch. 13 - Prob. 13.71CPCh. 13 - Prob. 13.72CPCh. 13 - Prob. 13.73CPCh. 13 - Prob. 13.74CPCh. 13 - Menthene, a compound found in mint plants, has the...Ch. 13 - Prob. 13.76CPCh. 13 - Prob. 13.77CPCh. 13 - Two products are possible when 2-pentene is...Ch. 13 - Ocimene, a compound isolated from the herb basil,...Ch. 13 - Describe how you could prepare the following...Ch. 13 - Which of the following compounds are capable of...Ch. 13 - Prob. 13.82GPCh. 13 - Superglue is an alkene polymer made from the...Ch. 13 - Draw all possible C5H10 alkene isomers having a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- An enzyme lowers the activation energy, (AG) of a reaction from 50.0 kcal/mol to 40.0 kcal/mol. Calulate the catalytic power at 310K. (R-1.987x10 kcal/mol)arrow_forwardDraw a typical axodendritic synapse, including a specific neurotransmitter of your choice, its associated postsynaptic receptors (indicating whether they are ionotropic or metabotropic), and any associated reuptake transporters or degradation enzymes. Please include a description of what specific steps would occur as an action potential reaches the axonal terminal.arrow_forwardGive a full arrow pushing mechanism of the spontaneous redox reaction between NAD+/NADH and oxaloacetate/malate. Please include diagram drawing of the mechanism! (Thank You!)arrow_forward
- 18. Which one of the compounds below is the major organic product obtained from the following series of reactions? 1. BH3 2. H2O2, NaOH H₂CrO4 CH2N2 oro ororos A B C D Earrow_forward17. Which one of the compounds below is the major organic product obtained from the following series of reactions? CI benzyl alcohol OH PBr3 Mg 1. CO2 SOCl2 ? ether 2. H+, H₂O CI Cl HO OH CI Cl A B C D Earrow_forward14. What is the IUPAC name of this compound? A) 6-hydroxy-4-oxohexanenitrile B) 5-cyano-3-oxo-1-pentanol C) 5-cyano-1-hydroxy-3-pentanone D) 1-cyano-5-hydroxy-3-pentanone E) 5-hydroxy-3-oxopentanenitrile HO. CNarrow_forward
- 13. What is the IUPAC name of this compound? A) 5-hydroxy-3,3-dimethylpentanoic acid B) 3,3-dimethylpentanoic acid C) 3,3-dimethyl-1-oxo-1,5-pentanediol D) 1,5-dihydroxy-3,3-dimethylpentanal E) 4-hydroxy-2,2-dimethylbutanoic acid HO OHarrow_forwardHelp me understand how carbon disulfide leads to toxicity in the brain, using terms like distal axonopathy, neurofilaments, covalent cross-linking, adducts, etc.,...please intuitively explain what is happening and where and the effects of it. For example, I know that CS2 reacts with amide and sulfhydryl groups on proteins, but what proteins exactly and where are they located?arrow_forwardWhat is the standard free energy change (in kJ/mole) of the spontaneous reaction between Oxygen and NADH to form H2O2 and NAD+?arrow_forward
- Redox Chemistry: Give standard free energy changes expected for the following reactions:-Succinate -> fumarate (using FAD/FADH2)-Oxaloacetate -> Malate (using NAD/NADH)-NADH --> NAD+ (using FMN/FMNH2)-CoQ --> CoQH2 (using Cytochrome C)arrow_forwardGive examples of balanced redox reactions that match the following:-Catabolic-Anabolic-Oxidative-Reductivearrow_forwardIf there are 20uM of a GLUT2 transporter on the surface of a cell, each able to move 8 per second, and 50mM glucose outside of the cell, what is the flux into the cell in mM/sec?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage


Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning

Essentials of Pharmacology for Health Professions
Nursing
ISBN:9781305441620
Author:WOODROW
Publisher:Cengage



GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license