
Concept explainers
A spring-loaded piston–cylinder device contains a mixture of gases whose pressure fractions are 25 percent Ne, 50 percent O2, and 25 percent N2. The piston diameter and spring are selected for this device such that the volume is 0.1 m3 when the pressure is 200 kPa and 1.0 m3 when the pressure is 1000 kPa. Initially, the gas is added to this device until the pressure is 200 kPa and the temperature is 10°C. The device is now heated until the pressure is 500 kPa. Calculate the total work and heat transfer for this process.
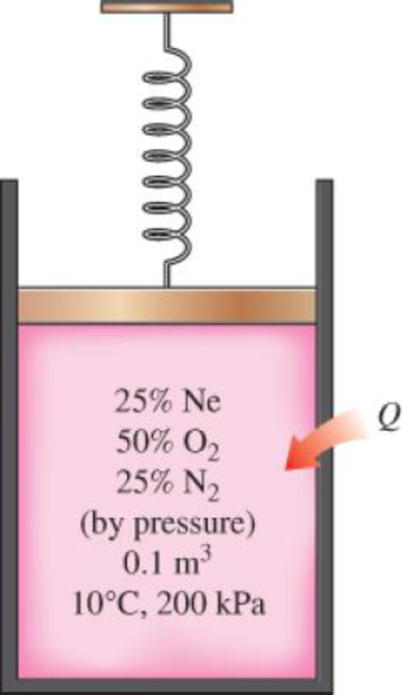
The total work done and heat transfer for the process.
Answer to Problem 93RP
The total work done for the process is
The heat transfer for the process is
Explanation of Solution
Write the expression to calculate the partial pressure of
Here, mixture pressure is
Write the expression to calculate the partial pressure of
Here, mixture pressure is
Write the expression to calculate the partial pressure of
Here, mixture pressure is
Write the expression to calculate the mass of
Write the expression to calculate the mass of
Write the expression to calculate the mass of
Write the expression to calculate the total mass of each component
Write the expression to calculate the mass fraction of
Write the expression to calculate the mass fraction of
Write the expression to calculate the mass fraction of
Write the expression to calculate the mole number of
Here, molar mass of
Write the expression to calculate the mole number of
Here, molar mass of
Write the expression to calculate the mole number of
Here, molar mass of
Write the expression to calculate the total number of moles
Write the expression to calculate the apparent molecular weight of the mixture
Write the expression to calculate the constant volume specific heat of the mixture
Here, mole fraction of
Write the expression to calculate the apparent gas constant of the mixture
Here, universal gas constant is
Write the expression for the mass contained in the system
Write the expression to calculate the final temperature
Write the expression to calculate the work done during the process.
Conclusion:
From Table A-1, “Molar mass, gas constant, and critical point properties”, obtain the values of molar masses for
From Table A-2a, “Ideal-gas specific heats of various common gases”, obtain the following properties for
For
For
For
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
The pressure changes linearly with volume as shown is Figure (1).
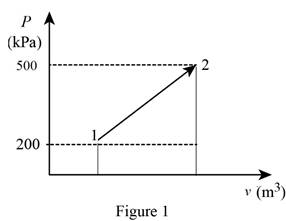
Using the data form Prob. 13–94 obtain the value of final volume by linear interpolation.
Write the straight line equation for two points.
Here, coordinates of the point 1 is
Substitute
The final volume
Substitute
Substitute
Thus, the total work done for the process is
Write a energy balance on the system.
Here, input energy transfer and output energy transfer is
The rate of change in energy of a system
For given system the energy balance Equation (XXII) is expressed as follows:
The rate of change in energy of a system
Substitute
Thus, the heat transfer for the process is
Want to see more full solutions like this?
Chapter 13 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- Solve this problem and show all of the workarrow_forwardSolve this problem and show all of the workarrow_forwarddraw the pneumatic circuit to operate a double-acting cylinder with: 1. Extension: Any of two manual conditions plus cylinder fully retracted, → Extension has both meter-in and meter-out, 2. Retraction: one manual conditions plus cylinder fully extended, → Retraction is very fast using quick exhaust valve.arrow_forward
- Correct answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you. Expert solution plsarrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forward
- Correct answer is written below. Detailed and complete solution only with fbd. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forward
- Correct answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution with fbd only. I will upvote, thank you.arrow_forwardCorrect answer is written below. Detailed and complete solution only. I will upvote, thank you.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





