
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
8th Edition
ISBN: 2818440043505
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.18, Problem 33P
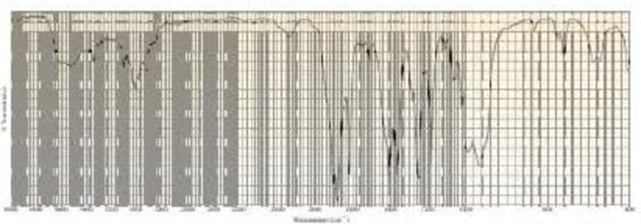
A compound with molecular formula C4H6O gives the infrared spectrum shown here. Identify the compound.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3) Catalytic hydrogenation of the compound below produced the expected product. However, a
byproduct with molecular formula C10H12O is also formed in small quantities. What is the by product?
What is the ΔHorxn of the reaction?
NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq)
ΔHorxn 1= ________ kJ/mol
=
+92kJ
ΔΗ
=
+170kJ
Use the following reactions:
2NH3(9) N2(g) + 3H2(g)
→
11/N2(g) + 2H2O (1) → NO2(g) + 2H2(g)
Determine the DH° of this reaction:
NO2(g) + H2(g)
→
2(g) → 2H2O(l) + NH3(9)
ΔΗ
Chapter 13 Solutions
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
Ch. 13.1 - Which of the following fragments produced in a...Ch. 13.2 - What distinguishes the mass spectrum of...Ch. 13.2 - What is the most likely m/z value for the base...Ch. 13.3 - Prob. 5PCh. 13.3 - a. Suggest possible molecular formulas for a...Ch. 13.3 - If a compound has a molecular ion with an...Ch. 13.3 - Identify the hydrocarbon that has a molecular ion...Ch. 13.4 - Predict the relative intensities of the molecular...Ch. 13.5 - Which molecular formula has an exact molecular...Ch. 13.5 - Prob. 11P
Ch. 13.6 - Sketch the mass spectrum expected for...Ch. 13.6 - The mass spectra of 1-methoxybutane,...Ch. 13.6 - Primary alcohols have a strong peak at m/z = 31....Ch. 13.6 - Identify the ketones responsible for the mass...Ch. 13.6 - Prob. 16PCh. 13.6 - Using curved arrows, show the principal fragments...Ch. 13.6 - The reaction of (Z)-2-pentene with water and a...Ch. 13.9 - a. Which is higher in energy: electromagnetic...Ch. 13.9 - Prob. 20PCh. 13.13 - Prob. 21PCh. 13.14 - Which occur at a larger wavenumber: a. the C O...Ch. 13.14 - Prob. 23PCh. 13.14 - Prob. 24PCh. 13.14 - Rank the following compounds from highest...Ch. 13.14 - Which shows an O H stretch at a larger...Ch. 13.16 - Prob. 27PCh. 13.16 - a. An oxygen-containing compound shows an...Ch. 13.16 - Prob. 29PCh. 13.16 - For each of the following pair of compounds, name...Ch. 13.17 - Which of the following compounds has a vibration...Ch. 13.17 - Prob. 32PCh. 13.18 - A compound with molecular formula C4H6O gives the...Ch. 13.20 - Prob. 34PCh. 13.20 - Prob. 35PCh. 13.21 - Predict the max of the following compound:Ch. 13.21 - Prob. 37PCh. 13.23 - a. At pH = 7 one of the ions shown here is purple...Ch. 13.23 - Prob. 39PCh. 13.23 - Prob. 40PCh. 13 - In the mass spectrum of the following compounds,...Ch. 13 - Prob. 42PCh. 13 - Draw structures for a saturated hydrocarbon that...Ch. 13 - Rank the following compounds in order of...Ch. 13 - For each of the following pairs of compounds,...Ch. 13 - a. How could you use IR spectroscopy to determine...Ch. 13 - Assuming that the force constant is approximately...Ch. 13 - Norlutin and Enovid are ketones that suppress so...Ch. 13 - In the following boxes, list the types of bonds...Ch. 13 - A mass spectrum shows significant peaks at m/z. =...Ch. 13 - Prob. 51PCh. 13 - Prob. 52PCh. 13 - Prob. 53PCh. 13 - The IR spectrum of a compound with molecular...Ch. 13 - Rank the following compounds from highest...Ch. 13 - Rank the following compounds from highest...Ch. 13 - What peaks in their mass spectra can be used to...Ch. 13 - Prob. 58PCh. 13 - Which one of the following five compounds produced...Ch. 13 - Prob. 60PCh. 13 - Each of the IR spectra shown below is accompanied...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - How can IR spectroscopy distinguish between...Ch. 13 - Prob. 65PCh. 13 - Prob. 66PCh. 13 - Give approximate wavenumbers for the major...Ch. 13 - Prob. 68PCh. 13 - Which one of the following live compounds produced...Ch. 13 - Phenolphthalein is an acid-base indicator. In...Ch. 13 - Prob. 71PCh. 13 - How can you use UV spectroscopy to distinguish...Ch. 13 - Prob. 73PCh. 13 - The IR and mass spectra for three different...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the entropy change for the reaction SO2(g) + O2(g) following information: Standard Entropy Values of Various Substance Substance SO2(g) 02(g) SO3(g) So (J/mol K) 248.2 205.0 256.8 → SO3(g) given thearrow_forwardIndicate which one of the following reactions most certainly results in a negative AS sys. O1402(g) + 3NH4NO3 (s) + C10 H22(1) → 3N2(g) + 17H2O(g) + 10CO2(g) ○ CO2(aq) = CO2(g) ○ H₂O(g) = H₂O(s) CaCO3(g) = CaO(s) + CO2(g) O CuSO4.5H2O(s) = CuSO4(s) + 5H2O(g)arrow_forwardEstimate the DH°rxn of the reaction below: H H-C-C=C-H H Н A table of bond energy Bond H Bond Energy (kJ/mol) C-H 413 C-O 360 C=O 743 C-C 348 |C = C 612 O-H 463 H-H 436 + H-H -> H H-C. - H | | 1 HHHarrow_forward
- Show work...don't give Ai generated solutionarrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 3A(g) + 1B (g) 4C (g) + 7D (g) Substance AH in kJ/mol A (g) - 25.07 B (g) - 36.51 C (g) - 90.09 D (g) + 56.11 AHran =?kJarrow_forwardWhat is the change in internal energy (ΔU) when a system is heated with 42.0 J of energy while it does 110.0 J of work?arrow_forward
- Can you help me solve this problem and explain what the answers are?arrow_forwardFor which reaction below does the enthalpy change under standard conditions correspond to a standard enthalpy of formation? (Choose all that applies) SO2(g) + 1/2 O2(g) → SO3(g) 2H2(g) + C(s) → CH4(g) Mg(s) + 1/2 O2(g) → MgO(s) CO(g) + H2O(g) → CO2(g) + H2(g) CO2(g) + H2(g) → CO(g) + H2O(g) 1/2 H2(g) + 1/2 N2(g) + 3/2 O2(g) → HNO3(g) CO2(g) + C(s) 2CO(g) N2(g) + 202(g) → 2NO2(g)arrow_forwardChoose all the molecules with zero standard-enthalpy-of-formation (AH% = 0) Fe(s) FeCl2(s) N2(g) H2O(l) 02(g) C(graphite) K(s) H2O(g)arrow_forward
- 8.5 g of potassium hydroxide (molar mass = 56.1 g/mol) dissolves in 125 g of water and the temperature of the solution increases by 15.58°C. Calculate the AH soln for potassium hydroxide. Assume the specific heat capacity of the solution is 4.2 J.g¨¹.ºC-1. KOH(s) → →K+ K(aq) + OH AH solution = ?kJ/mol (aq)arrow_forwardWhat will be the final temperature of a 8.79 g piece of iron (CP = 25.09 J/(mol · oC)) initially at 25.0oC, if it is supplied with 302.8 J from a stove?arrow_forwardIdentify the set of stoichiometric coefficients that balances the reaction equation for the combustion of the hydrocarbon below: _ C19 H4002 → CO2 + H2Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY