
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.14, Problem 13.32P
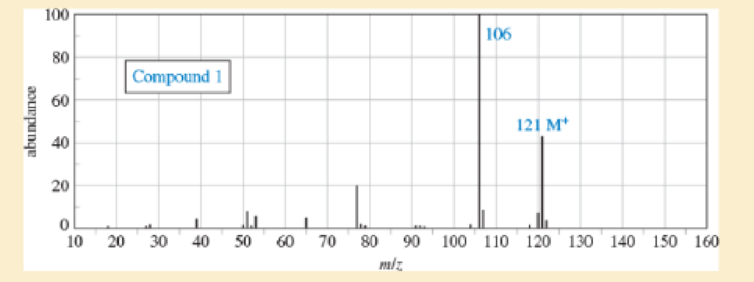
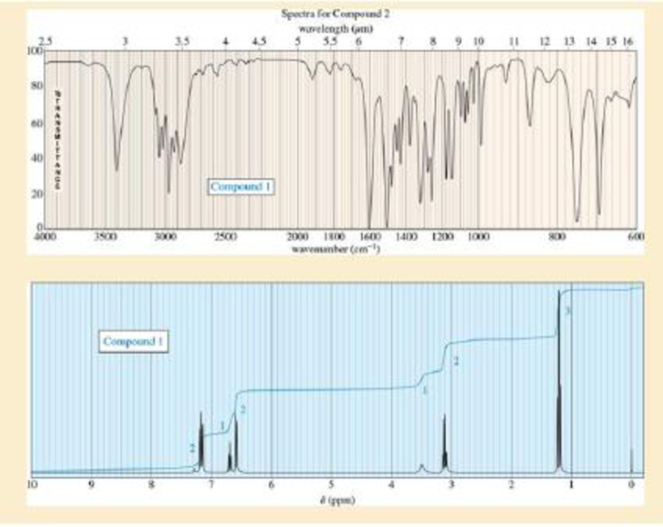
Sets of spectra are given for two compounds. For each set,
- A. look at each spectrum individually, and list the structural characteristics you can determine from that spectrum.
- B. look at the set of spectra as a group, and propose a tentative structure.
- C. verify that your proposed structure accounts for the major features of each spectrum. The soluton for compound 1 is given after the problem, but go as far as you can before looking at the solution.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting
at 32.0 °C?
What is the osmotic pressure (in atm) of a 1.46 M aqueous solution of urea [(NH2), CO] at 3
Round your answer to 3 significant digits.
Reagan is doing an atomic absorption experiment that requires a set of zinc standards in the 0.4-1.6 ppm range. A 1000 ppm Zn solution was prepared by dissolving the necessary amount of solid Zn(NO3)2 in water. The standards can be prepared by diluting the 1000 ppm Zn solution. Table 1 shows one possible set of serial dilutions (stepwise dilution of a solution) that Reagan could perform to make the necessary standards. Solution A was prepared by diluting 5.00 ml of the 1000 ppm Zn standard to 50.00 ml. Solutions C-E are called "calibration standards" because they will be used to calibrate the atomic absorption spectrometer.
a. Compare the solution concentrations expressed as ppm Zn and ppm Zn(NO3)2. Compare the concentrations expressed as M Zn and M Zn(NO3)2
- Which units allow easy conversion between chemical species (e.g. Zn and Zn(NO3)2)?
- Which units express concentrations in numbers with easily expressed magnitudes?
- Suppose you have an analyte for which you don't know the molar…
Chapter 13 Solutions
Organic Chemistry (9th Edition)
Ch. 13.5A - In a 300-MHz spectrometer, the protons in...Ch. 13.5B - Prob. 13.2PCh. 13.6 - Determine the number of different kinds of protons...Ch. 13.6 - Prob. 13.4PCh. 13.7 - Draw the integral trace expected for the NMR...Ch. 13.7 - Prob. 13.6PCh. 13.8C - Draw the NMR spectra you would expect for the...Ch. 13.8D - Draw the NMR spectra you expect for the following...Ch. 13.8D - a. Assign protons to the peaks in the NMR spectrum...Ch. 13.8D - Prob. 13.10P
Ch. 13.8D - Two spectra are shown. Propose a structure that...Ch. 13.9 - Prob. 13.12PCh. 13.9 - The spectrum of trans-hex-2-enoic acid follows. a....Ch. 13.9 - Prob. 13.14PCh. 13.9 - Prob. 13.15PCh. 13.10 - Prob. 13.16PCh. 13.10 - If the imaginary replacement of either of two...Ch. 13.10 - Predict the theoretical number of different NMR...Ch. 13.11B - Prob. 13.19PCh. 13.11B - Prob. 13.20PCh. 13.11B - Prob. 13.21PCh. 13.11B - Prob. 13.22PCh. 13.11B - Prob. 13.23PCh. 13.11B - Prob. 13.24PCh. 13.12E - Draw the expected broadband-decoupled 13 C N M R...Ch. 13.12E - a. Show which carbon atoms correspond with which...Ch. 13.12E - Repeat Problem13-25, sketching the...Ch. 13.12F - Prob. 13.28PCh. 13.13 - A bottle of allyl bromide was found to contain a...Ch. 13.13 - A laboratory student was converting cyclohexanol...Ch. 13.14 - Sets of spectra are given for two compounds. For...Ch. 13 - An unknown compound has the molecular formula C 9...Ch. 13 - Prob. 13.34SPCh. 13 - Predict the approximate chemical shifts of the...Ch. 13 - Prob. 13.36SPCh. 13 - Prob. 13.37SPCh. 13 - Prob. 13.38SPCh. 13 - Prob. 13.39SPCh. 13 - Prob. 13.40SPCh. 13 - For each compound shown below. 1. sketch the 13 C...Ch. 13 - Prob. 13.42SPCh. 13 - Prob. 13.43SPCh. 13 - Prob. 13.44SPCh. 13 - Prob. 13.45SPCh. 13 - Prob. 13.46SPCh. 13 - A compound was isolated as a minor constituent in...Ch. 13 - Prob. 13.48SPCh. 13 - The three isomers of dimethylbenzene are commonly...Ch. 13 - a. Draw all six isomers of formula C 4 H 8...Ch. 13 - Prob. 13.51SPCh. 13 - Hexamethylbenzene undergoes free-radical...Ch. 13 - Each of these four structures has molecular...Ch. 13 - Prob. 13.54SPCh. 13 - Phenyl Grignard reagent adds to 2-methylpropanal...Ch. 13 - Prob. 13.56SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CH₂O and 22 NMR Solvent: CDCl3 IR Solvent: neat 4000 3000 2000 1500 1000 15 [ اند 6,5 9.8 3.0 7.0 6.0 5.0 4.8 3.0 2.0 1.0 9.8 200 100arrow_forwardprotons. Calculate the mass (in grams) of H3AsO4 (MW=141.9416) needed to produce 3.125 x 1026arrow_forwardPlease provide with answer, steps and explanation of ideas to solve.arrow_forward
- Please provide with answer, steps and explanation of ideas to solve.arrow_forwardPlease provide with answer, steps and explanation of ideas to solve.arrow_forwardUsing what we have learned in CHEM 2310 and up through class on 1/31, propose a series of reaction steps to achieve the transformation below. Be sure to show all reagents and intermediates for full credit. You do not need to draw mechanism arrows, but you do need to include charges where appropriate. If you do not put your group name, you will get half credit at most. ? Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY