
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
thumb_up100%
Chapter 13, Problem 99QAP
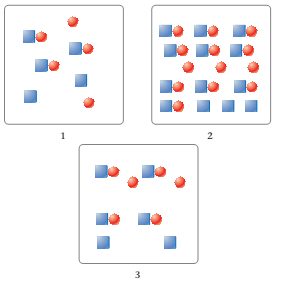
Each box represents an acid solution at equilibrium. Squares represent H+ ions. Circles represent anions. (Although the anions have different identities in each figure, they are all represented as circles.) Water molecules are not shown. Assume that all solutions have the same volume.
(a) Which figure represents the strongest acid?
(b) Which figure represents the acid with the smallest Ka?
(c) Which figure represents the acid with the lowest pH?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 13 Solutions
Chemistry: Principles and Reactions
Ch. 13 - For each of the following reactions, indicate the...Ch. 13 - Follow the direction for Question 1 for the...Ch. 13 - According to the Brønsted-Lowry theory, which of...Ch. 13 - According to the Brønsted-Lowry theory, which of...Ch. 13 - Give the formula of the conjugate acid of (a) OH-...Ch. 13 - Give the formula for the conjugate base of (a)...Ch. 13 - Write a balanced equation showing how the H2PO4-...Ch. 13 - Follow the instructions of Question 7 for the...Ch. 13 - Using the Brønsted-Lowry model, write equations...Ch. 13 - Prob. 10QAP
Ch. 13 - Using the Brønsted-Lowry model, write an equation...Ch. 13 - Prob. 12QAPCh. 13 - Find the pH of solutions with the following[ H+ ]....Ch. 13 - Find the pH of solutions with the following[ H+ ]....Ch. 13 - Calculate H+ and OH- and in solutions with the...Ch. 13 - Calculate [H+] and [OH-] in solutions with the...Ch. 13 - Complete the following table for solutions at 25C.Ch. 13 - Complete the following table for solutions at 25C.Ch. 13 - Solution 1 has [ H+ ]=1.7102 M. Solution 2 has [...Ch. 13 - Solution R has pH 13.42. Solution Q has [ OH...Ch. 13 - Consider three solutions, R, Z, and Q. •...Ch. 13 - Solution A has a pH of 12.32. Solution B has [H+]...Ch. 13 - Unpolluted rain water has a pH of about 5.5. Acid...Ch. 13 - Most cola soft drinks have a pH of 3.1. Green tea...Ch. 13 - Find [OH-] and the pH of the following solutions....Ch. 13 - Find [H+] and the pH of the following solutions....Ch. 13 - Find [OH+], [OH-] and the pH of the following...Ch. 13 - Find [OH-], [H+], and the pH of the following...Ch. 13 - How many grams of HI should be added to 265 mL of...Ch. 13 - What is the pH of a solution obtained by adding...Ch. 13 - What is the pH of a solution obtained by adding...Ch. 13 - What is the pH of a solution obtained by mixing...Ch. 13 - Write the ionization equation and the Ka for each...Ch. 13 - Write the ionization equation and the Ka...Ch. 13 - Calculate Ka for the weak acids that have the...Ch. 13 - Prob. 36QAPCh. 13 - Prob. 37QAPCh. 13 - Consider these acids (a) Arrange the acids in...Ch. 13 - Rank the following solutions in order of...Ch. 13 - Rank the following acids (M=0.10)in order of...Ch. 13 - Prob. 41QAPCh. 13 - Rank the solutions in Questions 40 in order of...Ch. 13 - The pH of a 0.129 M solution of a weak acid, HB,...Ch. 13 - The pH of a 2.642 M solution of a weak acid, HB,...Ch. 13 - Paraminobenzene (PABA), HC7H6NO2, is used in some...Ch. 13 - Acetaminophen, HC8H8NO2 (MM=151.17g/mol), is the...Ch. 13 - Caproic acid, HC6H11O2, is found in coconut oil...Ch. 13 - Barbituric acid, HC4H3N2O3, is used to prepare...Ch. 13 - When aluminum chloride dissolves in water,...Ch. 13 - Using the Ka values in Table 13.2, calculate the...Ch. 13 - Barbituric acid, HC4H3N2O3, is used to prepare...Ch. 13 - Penicillin(MM=356g/mol), an antibiotic often used...Ch. 13 - Gallic acid, HC7H5O5, an ingredient in some...Ch. 13 - Anisic acid (K a=3.38105) is found in anise seeds...Ch. 13 - Phenol, once known as carbolic acid, HC6H5O, is a...Ch. 13 - Benzoic acid (K a=6.6105)is present in many...Ch. 13 - Chromic acid, H2CrO4, is commonly obtained by...Ch. 13 - Consider citric acid, H3C6H5O7, added to many soft...Ch. 13 - Consider a 0.45 M solution of ascorbic...Ch. 13 - Consider a 0.33 M solution of the diprotic acid...Ch. 13 - Phthalic acid H2C8H4O4, is a diprotic acid. It is...Ch. 13 - Selenious acid, H2SeO3, is primarily used to...Ch. 13 - Write the ionization expression and the Kb...Ch. 13 - Follow the instructions for Question 63 for the...Ch. 13 - Prob. 65QAPCh. 13 - Follow the directions of Question 65 for the...Ch. 13 - Using the equilibrium constants listed in Table...Ch. 13 - Using the equilibrium constants listed in Table...Ch. 13 - Find the value of Kb for the conjugate base of the...Ch. 13 - Find the values of Kb for the conjugate bases of...Ch. 13 - Determine [OH-], pOH and pH of a 0.28 M aqueous...Ch. 13 - Determine the [OH-] and pH of a 0.72 M solution of...Ch. 13 - Codeine (Cod), a powerful and addictive...Ch. 13 - Consider pyridine, C5H5N, a pesticide and deer...Ch. 13 - A solution of baking soda, NaHCO3, has a pH of...Ch. 13 - A solution of sodium cyanide, NaCN, has a pH of...Ch. 13 - Write formulas for two salts that (a) contain Ni3+...Ch. 13 - Write formulas for two salts that (a) contain NH4+...Ch. 13 - State whether 1 M solutions of the following salts...Ch. 13 - State whether 1 M solutions of the following salts...Ch. 13 - Write net ionic equations to explain the acidity...Ch. 13 - Prob. 82QAPCh. 13 - Arrange the following aqueous 0.1 M solutions in...Ch. 13 - Arrange the following aqueous 0.1 M solutions in...Ch. 13 - Unclassified At 25C, a 0.20 M solution of...Ch. 13 - Prob. 86QAPCh. 13 - There are 324 mg of acetylsalicylic acid...Ch. 13 - A student is asked to bubble enough ammonia gas...Ch. 13 - Prob. 89QAPCh. 13 - A student prepares 455 mL of a KOH solution, but...Ch. 13 - Consider the process H2O H+(aq)+OH(aq)H=55.8kJ (a)...Ch. 13 - Household bleach is prepared by dissolving...Ch. 13 - A tablet with a mass of 4.08 g contains 71.2%...Ch. 13 - Consider a weak organic base (nonelectrolyte) with...Ch. 13 - Prob. 95QAPCh. 13 - Which of the following is/are true regarding a 0.1...Ch. 13 - Which of the following is/are true about a 0.10 M...Ch. 13 - Consider the following six beakers. All have 100...Ch. 13 - Each box represents an acid solution at...Ch. 13 - Each box represents an acid solution at...Ch. 13 - Prob. 101QAPCh. 13 - You are asked to determine whether an unknown...Ch. 13 - What is the pH of a 0.020 M solution of H2SO4? You...Ch. 13 - Prob. 104QAPCh. 13 - What is the pH of a solution obtained by mixing...Ch. 13 - A solution is made up of 273 mL of 0.164 M HNO3...Ch. 13 - What is the freezing point of vinegar, which is an...Ch. 13 - Prob. 108QAPCh. 13 - Consider two weak acids, HA (MM=138g/mol)and HB...Ch. 13 - Consider an aqueous solution of a weak base, NaB...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY