
(a)
Interpretation: The boiling point and freezing point of the given solution of glucose in ethanol should be determined.
Concept Introduction:
The elevation in boiling point of the ethanol is calculated by using the formula,
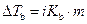
Where,
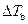 is the elevation in boiling point.
is the elevation in boiling point.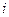 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.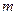 is the molality of the solution.
is the molality of the solution.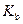 is the molal boiling point elevation constant
is the molal boiling point elevation constant 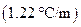 refer table 13.3)
refer table 13.3)
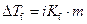
Where,
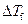 is the depression in freezing point.
is the depression in freezing point.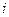 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.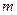 is the molality of the solution.
is the molality of the solution.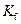 is the molal freezing point depression constant
is the molal freezing point depression constant 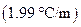 refer table13.3)
refer table13.3)
(b)
Interpretation: The boiling point and freezing point of the given solution of decane in chloroform should be calculated.
Concept Introduction:
The elevation in boiling point of the ethanol is calculated by using the formula,
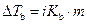
Where,
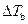 is the elevation in boiling point.
is the elevation in boiling point.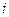 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.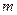 is the molality of the solution.
is the molality of the solution.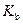 is the molal boiling point elevation constant
is the molal boiling point elevation constant 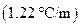 refer table 13.3)
refer table 13.3)
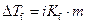
Where,
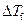 is the depression in freezing point.
is the depression in freezing point.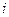 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.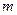 is the molality of the solution.
is the molality of the solution.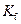 is the molal freezing point depression constant
is the molal freezing point depression constant 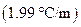 refer table13.3)
refer table13.3)
(c)
Interpretation: The boiling point and freezing point of the given solution of 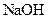 in water should be calculated.
in water should be calculated.
Concept Introduction:
The elevation in boiling point of the ethanol is calculated by using the formula,
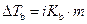
Where,
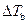 is the elevation in boiling point.
is the elevation in boiling point.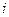 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.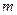 is the molality of the solution.
is the molality of the solution.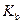 is the molal boiling point elevation constant
is the molal boiling point elevation constant 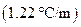 refer table 13.3)
refer table 13.3)
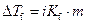
Where,
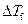 is the depression in freezing point.
is the depression in freezing point.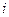 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.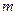 is the molality of the solution.
is the molality of the solution.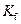 is the molal freezing point depression constant
is the molal freezing point depression constant 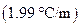 refer table13.3)
refer table13.3)
(d)
Interpretation: The boiling point and freezing point of the given solution of ethylene glycol and 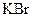 in water should be calculated.
in water should be calculated.
Concept Introduction:
The elevation in boiling point of the ethanol is calculated by using the formula,
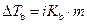
Where,
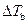 is the elevation in boiling point.
is the elevation in boiling point.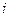 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.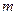 is the molality of the solution.
is the molality of the solution.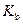 is the molal boiling point elevation constant
is the molal boiling point elevation constant 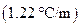 refer table 13.3)
refer table 13.3)
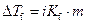
Where,
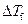 is the depression in freezing point.
is the depression in freezing point.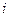 is the van’t Hoff factor which describes the number of ions of solute particles in the solution.
is the van’t Hoff factor which describes the number of ions of solute particles in the solution.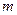 is the molality of the solution.
is the molality of the solution.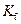 is the molal freezing point depression constant
is the molal freezing point depression constant 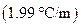 refer table13.3)
refer table13.3)
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
CHM 101 VOL 1 2014 >IC<
- Predict the product of this organic reaction: O OH + H + OH A P + H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click and drag to start drawing a structure. X G ☐ :arrow_forward0.0994 g of oxalic acid dihydrate is titrated with 10.2 mL of potassium permanganate. Calculate the potassium permanganate concentration. Group of answer choices 0.0433 M 0.135 M 0.0309 M 0.193 Marrow_forwardExperts...can any one help me solve these problems?arrow_forward
- According to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forwardWhich Group 1 metal reacts with O2(g) to form a metal peroxide (M2O2)? Group of answer choices Li K Rb Naarrow_forwardWhich of the following statements is true regarding the reaction between Group 1 metals and water? Group of answer choices These reactions result in a basic solution. The metals do not actually react easily with water due to the metals' lack of conductivity. These reaction result in an acidic solution. The metals need their outer coatings of metal oxides to react.arrow_forward
- Which element cannot interact with hydrogen through hydrogen bonds? Group of answer choices O S Br Narrow_forwardWhich of the following statements is false regarding hydrogen gas production? Group of answer choices Steam reforming requires a catalyst. Methanol (CH3OH) can react with water using a ZnO catalyst to form H2(g). Methanol (CH3OH) can react with O2(g) using a Pd catalyst to form H2(g). The reaction between CH4(g) and H2O to form H2(g) requires a temperature of at least 700 oCarrow_forwardWhich of the following forms of hydrogen is the least stable? Group of answer choices H H2 H− H+arrow_forward
- Consider the following reduction half reactions and standard reduction potentials: Fe3+ + e− → Fe2+ Eo = +0.77 V Fe2+ + e− → Fe(s) Eo = -0.44 V Which of the following statements is true? Group of answer choices The Fe2+ reduction to Fe(s) is spontaneous. Fe2+ can disproportionate into Fe3+ and Fe(s) The Fe3+ reduction to Fe2+ is not spontaneous. Fe3+ and Fe(s) can undergo a comproportionation reaction to form Fe2+arrow_forwardAccording to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forwardConsider the redox reaction: 2 P4 + 8 OH− + 4 H2O → 4 PH3 + 4 HPO32− The element oxidized is ["", "", ""] , the element reduced is ["", "", ""] , one of the oxidizing agents is ["", "", ""] , and the reducing agent is ["", "", ""] .arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





