
ORGANIC CHEMISTRY BOOK& SG/SM
6th Edition
ISBN: 9781264094493
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 65P
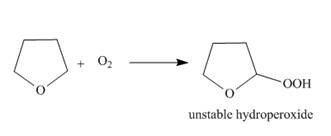
Ethers are oxidized with
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
reaction scheme for C39H4202
Hydrogenation of Alkyne (Alkyne to
Alkene)
show reaction (drawing) please
Give detailed mechanism Solution with explanation needed. Don't give Ai generated solution
Show work with explanation needed....don't give Ai generated solution
Chapter 13 Solutions
ORGANIC CHEMISTRY BOOK& SG/SM
Ch. 13.1 - Prob. 1PCh. 13.1 - Prob. 2PCh. 13.2 - Prob. 3PCh. 13.3 - Prob. 4PCh. 13.3 - Prob. 5PCh. 13.4 - Prob. 7PCh. 13.5 - Problem 15.8 Which bond in the each compound is...Ch. 13.6 - Prob. 9PCh. 13.6 - Prob. 10PCh. 13.7 - Prob. 11P
Ch. 13.7 - Prob. 12PCh. 13.8 - Prob. 13PCh. 13.8 - Prob. 14PCh. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 34PCh. 13 - 15.37 What alkane is needed to make each alkyl...Ch. 13 - 15.38 Which alkyl halides can be prepared in good...Ch. 13 - Prob. 37PCh. 13 - 15.40 Explain why radical bromination of p-xylene...Ch. 13 - a. What product(s) (excluding stereoisomers) are...Ch. 13 - Prob. 40PCh. 13 - 15.43 Draw the products formed when each alkene is...Ch. 13 - 15.44 Draw all constitutional isomers formed when...Ch. 13 - 15.45 Draw the organic products formed in each...Ch. 13 - Prob. 45PCh. 13 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 13 - 15.48 Draw the products formed in each reaction...Ch. 13 - 15.53 Consider the following bromination: .
a....Ch. 13 - 15.54 Draw a stepwise mechanism for the following...Ch. 13 - Prob. 57PCh. 13 - 15.57 Devise a synthesis of each compound from...Ch. 13 - Prob. 59PCh. 13 - Prob. 60PCh. 13 - 15.60 Devise a synthesis of each compound using ...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - 15.63 As described in Section 9.16, the...Ch. 13 - 15.64 Ethers are oxidized with to form...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY