
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 42P
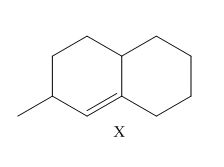
Draw all constitutional isomers formed when X is treated with
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Ethers can be formed via acid-catalyzed acetal formation. Draw the mechanism for the molecule below and ethanol.
HOCH,
H
HO
CH-OH
OH H
OH
11
CH₂OH
F
II
OH H
H
0
+
H OH
Draw the mechanism for the formation of diol by starting with one pen and all in...
basic conditions
then
acidic conditions
then draw the mechanism for the formation of a carboxylic acid from your product.
Chapter 13 Solutions
ORGANIC CHEMISTRY
Ch. 13.1 - Prob. 1PCh. 13.1 - Prob. 2PCh. 13.2 - Prob. 3PCh. 13.3 - Prob. 4PCh. 13.3 - Prob. 5PCh. 13.4 - Prob. 7PCh. 13.5 - Problem 15.8 Which bond in the each compound is...Ch. 13.6 - Prob. 9PCh. 13.6 - Prob. 10PCh. 13.7 - Prob. 11P
Ch. 13.7 - Prob. 12PCh. 13.8 - Prob. 13PCh. 13.8 - Prob. 14PCh. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 34PCh. 13 - 15.37 What alkane is needed to make each alkyl...Ch. 13 - 15.38 Which alkyl halides can be prepared in good...Ch. 13 - Prob. 37PCh. 13 - 15.40 Explain why radical bromination of p-xylene...Ch. 13 - a. What product(s) (excluding stereoisomers) are...Ch. 13 - Prob. 40PCh. 13 - 15.43 Draw the products formed when each alkene is...Ch. 13 - 15.44 Draw all constitutional isomers formed when...Ch. 13 - 15.45 Draw the organic products formed in each...Ch. 13 - Prob. 45PCh. 13 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 13 - 15.48 Draw the products formed in each reaction...Ch. 13 - 15.53 Consider the following bromination: .
a....Ch. 13 - 15.54 Draw a stepwise mechanism for the following...Ch. 13 - Prob. 57PCh. 13 - 15.57 Devise a synthesis of each compound from...Ch. 13 - Prob. 59PCh. 13 - Prob. 60PCh. 13 - 15.60 Devise a synthesis of each compound using ...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - 15.63 As described in Section 9.16, the...Ch. 13 - 15.64 Ethers are oxidized with to form...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill-in-the molecules for the oxidation or reduction of the starting alcohol.arrow_forwardName the following carbohydrates give both the systematic and common names. Don't forget to identify the Isomer.arrow_forwardWhat is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY