
Concept explainers
(a)
Interpretation:
The following conversion should be explained.
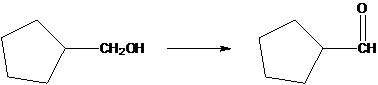
Concept Introduction:
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an
(b)
Interpretation:
The following conversion should be explained.

Concept Introduction:
Chemical reaction is the procedure to transform the chemical substance in the new substance. During this procedure sometimes, acid is involved which is the substance which could either accept electron pairs or donate protons in the reactions.
A catalyst is the molecule which is used to speed up the chemical reaction, not being consumed within the procedure. Acids are used as catalysts are needed for an acid catalyzed hydration.
A dehydration reaction is aeration when any organic substance loses the water molecule to form an alkene.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Introduction to General, Organic and Biochemistry
- please draw and example of the following: Show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardNaming and drawing secondary Write the systematic (IUPAC) name for each of the following organic molecules: CH3 Z structure CH3 CH2 CH2 N-CH3 CH3-CH2-CH2-CH-CH3 NH CH3-CH-CH2-CH2-CH2-CH2-CH2-CH3 Explanation Check ☐ name ☐ 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C Garrow_forwardC This question shows how molecular orbital (MO) theory can be used to understand the chemical properties of elemental oxygen O₂ and its anionic derivative superoxide Oz. a) Draw the MO energy diagram for both O2 and O2. Clearly label your diagram with atomic orbital names and molecular orbital symmetry labels and include electrons. Draw the Lewis structure of O2. How does the MO description of O2 differ from the Lewis structure, and how does this difference relate to the high reactivity and magnetic properties of oxygen? ) Use the MO diagram in (a) to explain the difference in bond length and bond energy between superoxide ion (Oz, 135 pm, 360 kJ/mol) and oxygen (O2, 120.8 pm, 494 kJ/mol).arrow_forward
- Please drawarrow_forward-Page: 8 nsition metal ions have high-spin aqua complexes except one: [Co(HO)₁]". What is the d-configuration, oxidation state of the metal in [Co(H:O))"? Name and draw the geometry of [Co(H2O)]? b) Draw energy diagrams showing the splitting of the five d orbitals of Co for the two possible electron configurations of [Co(H2O)]: Knowing that A = 16 750 cm and Пl. = 21 000 cm, calculate the configuration energy (.e., balance or ligand-field stabilization energy and pairing energy) for both low spin and high spin configurations of [Co(H2O)]. Which configuration seems more stable at this point of the analysis? (Note that 349.76 cm = 1 kJ/mol) Exchange energy (IT) was not taken into account in part (d), but it plays a role. Assuming exchange an occur within t29 and within eg (but not between tz, and ea), how many exchanges are possible in the low in configuration vs in the high spin configuration? What can you say about the importance of exchange energy 07arrow_forwardDraw everything please on a piece of paper explaining each steparrow_forward
- Define crystalline, polycrystalline and amorphous materials What crystal system and Bravais lattices are shown in the figure immediately below? What do a, b, C, a, ẞ and y represent and what are their values? You can label the Bravais lattices directly above or under the figure. C aarrow_forward32. The diagrams below show the band structure of an intrinsic semiconductor at absolute zero and room temperature. Room Temperature EF E OK Ep- a) In the space below, sketch a similar pair of diagrams for an n-type semiconductor. D) Give the definition and an example of (i) an intrinsic semiconductor and (ii) an n-type semiconductor.arrow_forward29. a) i Which energy diagram best represents the d-electrons in tetrahedral [Co(NH3)4]²+? b) ii c) iii d) iv 11 ་ ↑↓ ↑t t ↑↓ ↑↓ e) none of these ii In1 According to Slater's rules, what is the effective nuclear charge experienced by a 3d electron in 30. Ge? a) 32.00 b) 21.15 c) 16.05 d) 14.00 e) 10.85arrow_forward
- Regarding Lowis structuros and geometrios, Draw Lewis structures for the following: SOF4, SO, ICI, XeO2F4, SeF and XeO3. For each one, indicate the observed molecular geometry it adopts.arrow_forwardExplain the following statements with equations: - The fusion product of an organic compund with sodium metal is an alkaline solution - The test for elements should be done before the solubility tests. - Using less sodium than the organic compound in the ignition test might cause a problem to detect the presence of the nitrogen and sulfur - Formation of colored product when adding ferric acid chloride to phenol solutionarrow_forward31 Indicate the symbol, mass number and the atomic number of the missing product in each of the following nuclear reactions. a) 13/3 N 41 b) 11 Ca 20 c) 90 38 Sr → 133 C + ? + - 6 0 e →? 90 Y + ? 39 11 d) 22 Na → ? + + 1 B +1 β Toarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
