
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
3rd Edition
ISBN: 9781118203774
Author: Klein
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Question
Chapter 13, Problem 28PP
Interpretation Introduction
Interpretation: To construct all the possible constitutional isomers of ether for the molecular formula
Concept Introduction:
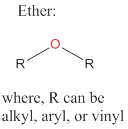
Ethers: Organic compounds that exhibit an oxygen atom bonded to two R groups, where each R groups can be an alkyl, aryl, or vinyl group.
HDI calculation: The HDI value calculated from the molecular formula is used to predict the degree of unsaturation in the structure.
To identify: construct all the possible constitutional isomers of a molecular formula
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider the following nucleophilic substitution reaction. The compound listed above the arrow is the solvent for the reaction. If nothing is listed over the arrow,
then the nucleophile is also the solvent for the reaction.
Part 1 of 2
Br
CH,CN
+ I¯
What is the correct mechanism for the reaction? Select the single best answer.
@SN2
○ SN 1
Part: 1/2
Part 2 of 2
Draw the products for the reaction. Include both the major organic product and the inorganic product. If more than one stereoisomer is possible, draw
only one stereoisomer. Include stereochemistry where relevant.
Click and drag to start drawing a
structure.
X
હૈ
20.33 Think-Pair-Share
(a) Rank the following dienes and dienophiles in order of increasing reactivity in the
Diels-Alder reaction.
(i)
CO₂Et
(ii)
COEt
||
CO₂Et
MeO
MeO
(b) Draw the product that results from the most reactive diene and most reactive
dienophile shown in part (a).
(c) Draw a depiction of the orbital overlap involved in the pericyclic reaction that oc-
curs between the diene and dienophile in part (b).
(d) Is the major product formed in part (b) the endo or exo configuration? Explain
your reasoning.
20.40 The following compound undergoes an intramolecular Diels-Alder reaction to give a
tricyclic product. Propose a structural formula for the product.
CN
heat
An intramolecular
Diels-Alder adduct
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
Ch. 13.2 - Prob. 1LTSCh. 13.2 - Prob. 1PTSCh. 13.2 - Prob. 2PTSCh. 13.2 - Prob. 3ATSCh. 13.4 - Prob. 4CCCh. 13.5 - Prob. 2LTSCh. 13.5 - PRACTICE the skill
Show reagents that you could...Ch. 13.5 -
APPLY the skill
Compound 2 was made as a...Ch. 13.5 - Prob. 7CCCh. 13.5 - Prob. 8CC
Ch. 13.5 - Prob. 9CCCh. 13.6 - Prob. 10CCCh. 13.7 - Prob. 11CCCh. 13.7 - Prob. 12CCCh. 13.8 - Prob. 3LTSCh. 13.8 - Prob. 13PTSCh. 13.8 - Prob. 14ATSCh. 13.9 - Prob. 15CCCh. 13.10 - Prob. 4LTSCh. 13.10 - Prob. 16PTSCh. 13.10 - Prob. 17ATSCh. 13.10 - Prob. 5LTSCh. 13.10 - Prob. 18PTSCh. 13.10 -
The sequence below shows an enantioselective...Ch. 13.11 - Prob. 20CCCh. 13.11 - Prob. 21CCCh. 13.12 - Prob. 6LTSCh. 13.12 - Prob. 22PTSCh. 13.12 - Prob. 23ATSCh. 13.12 - Prob. 7LTSCh. 13.12 - Prob. 24PTSCh. 13.12 - Prob. 25ATSCh. 13 - Prob. 26PPCh. 13 - Prob. 27PPCh. 13 - Prob. 28PPCh. 13 - Prob. 29PPCh. 13 - Prob. 30PPCh. 13 - Prob. 31PPCh. 13 - Prob. 32PPCh. 13 - Methylmagnesium bromide reacts rapidly with...Ch. 13 - Prob. 34PPCh. 13 - Prob. 35PPCh. 13 - Prob. 36PPCh. 13 - Prob. 37PPCh. 13 - Prob. 38PPCh. 13 - Prob. 39PPCh. 13 - Prob. 40PPCh. 13 - Prob. 41PPCh. 13 - Prob. 42PPCh. 13 - Prob. 43PPCh. 13 - Prob. 44PPCh. 13 - Prob. 45PPCh. 13 - Prob. 46IPCh. 13 - Prob. 47IPCh. 13 - Prob. 48IPCh. 13 - Prob. 49IPCh. 13 - Prob. 50IPCh. 13 - Prob. 51IPCh. 13 - Prob. 52IPCh. 13 - Prob. 53IPCh. 13 - Prob. 54IPCh. 13 - Prob. 55IPCh. 13 - Prob. 56IPCh. 13 - Prob. 57IPCh. 13 - Prob. 58IPCh. 13 - Prob. 59IPCh. 13 - Prob. 60IPCh. 13 - Prob. 61IPCh. 13 - Prob. 62IPCh. 13 - Prob. 63IPCh. 13 - Prob. 64IPCh. 13 - Prob. 65IPCh. 13 - Prob. 66IPCh. 13 - Prob. 67IPCh. 13 - Prob. 68IPCh. 13 - Prob. 69IPCh. 13 - Prob. 70IPCh. 13 - The following procedure13 is part of a synthetic...Ch. 13 - Prob. 72CPCh. 13 - Prob. 73CPCh. 13 - Prob. 74CPCh. 13 - Prob. 75CP
Knowledge Booster
Similar questions
- What is the reaction mechanism for this?arrow_forwardWhat is the reaction mechanism for this?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. + Drawing Arrows CH3ONA, CH3OH heat : Br:O Na → H H Br Na + H H H H H :0: .H + Undo Reset Done Q CH3 Drag To Pan +arrow_forward
- What is the reaction mechanism for this?arrow_forward20.19 Predict the structure of the major 1,2-addition product formed by reaction of one mole of Cl₂ with 3-methylenecyclohexene. Also predict the structure of the 1,4-addition product formed under these conditions. 20.20 Which of the two molecules shown do you expect to be the major product formed by 1,2-addition of HCI to cyclopentadiene? Explain. Cyclopentadiene + HC 3-Chlorocyclopentene (racemic) or 4-Chlorocyclopentene (racemic)arrow_forward20.35 Propose structural formulas for compounds A and B and specify the configuration of compound B. EtO₂C 250°C C14H2004 CO₂Et 1. Oso, then NaHSO3 2. HIO4 C14H2006 A Barrow_forward
- 20.21 Predict the major product formed by 1,4-addition of HCI to cyclopentadiene. 20.22 Draw structural formulas for the two constitutional isomers with the molecular for- mula C₂H,Br, formed by adding one mole of Br, to cyclopentadiene.arrow_forwardAdd substituents to draw the conformer below (sighting down the indicated bond), then rotate the back carbon to provide the conformation that will be capable of an E2 elimination. R/S stereochemistry is graded. + I I H CH3 Ph Досн Br OCH 3 Drawing Q H Atoms, Bonds and Rings Charges Tap a node to see suggestions. H H H H H Undo Reset Remove Done Rotatearrow_forward20.17 Predict the structure of the major product formed by 1,2-addition of HBr to 3-methylenecyclohexene. 3-Methylenecyclohexene 20.18 Predict the major product formed by 1,4-addition of HBr to 3-methylenecyclohexene.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY