
Concept explainers
Interpretation:
Detailed mechanism of hydrobromination via 1,2-addition and 1,4-addition mechanisms using peroxide promoted addition to 1,3-butadiene are to be written.
Concept introduction:
舧 Electrophiles are electron-deficient species, which has positive or partially positive charge. Lewis acids are electrophiles, which accept electron pair.
舧 Nucleophiles are electron rich species, which has negative or partially negative charge. Lewis bases are nucleophiles, which donate electron pair.
舧 Free radical is an atom, molecule, or ion that has an unpaired electron, which makes it highly chemically reactive.
舧 Substitution reaction: A reaction in which one of the hydrogen atoms of a hydrocarbon or a
舧 Elimination reaction: A reaction in which two substituent groups are detached and a double bond is formed is called elimination reaction.
舧 Addition reaction: It is the reaction in which unsaturated bonds are converted to saturated molecules by the addition of molecules.
舧 The reaction in which there is addition of hydrogen molecule is called hydrogenation reaction.
舧
舧 Hydrogenation with platinum as a catalyst is used to convert unsaturated carbohydrates to saturated hydrocarbons
舧 Oxidation of
舧 Ozonolysis helps convert the carbon–carbon double bonds to carbon–oxygen double bond (carbonyl compounds).
舧 Dimethyl sulfide is used as a reducing agent that decomposes the intermediate formed into the carbonyl group.
舧 NBS (nitro-bromo succinimide) is a special reagent used for bromination of allylic carbocations.
舧 Bromine replaces the hydrogen attached to the carbon adjacent to the carbon bearing double bond.
舧 This method of using NBS can produce allylic bromides without bromine reacting with the double bond.
舧 Dehydration of a primary alcohol in the presence of a mineral acid like concentrated sulfuric acid results in the formation of alkene via E2 elimination
舧 The 1,2–addition to a diene is addition of an electrophile to the carbon designated as 1 and a nucleophile to the carbon designated as 2. The positions of carbons as 1 and 2 are not according to the IUPAC numbering of the molecule, but as a conjugated diene molecule. 1,4-addition results in addition of hydrogen to the carbon designated as 1 and a halogen to the carbon designated as 4. The mechanism of 1,2 addition and 1,4-addition of hydro halogenation is given below.
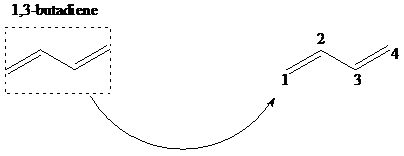

舧 Organic peroxides are used in free radical chain reactions in the initiation steps.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
ORGANIC CHEM. VOL.1+2-W/WILEYPLUS
- 10. The most important reason why Br- is a better nucleophile than Cl-is ___. A. polarizability; B. size; C. solvation; D. basicity; E. polarity. Please include all steps. Thanks!arrow_forwardPredicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid Ка K₁₁ name formula name formula nitrous acid HNO2 4.5×10 4 pyridine CHEN 1.7 × 10 9 4 hydrofluoric acid HF 6.8 × 10 methylamine CH3NH2 | 4.4 × 10¯ Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution 0.1 M NaNO2 0.1 M KF pH choose one v choose one v 0.1 M C5H5NHBr 0.1 M CH3NH3CI choose one v ✓ choose one 1 (lowest) 2 ☑ 3 4 (highest) 000 18 Ararrow_forward4. The major product from treatment of 2-propanol with the Jonesreagent is ___.A. acetone; B. none of the other answers is correct C. propene; D.propanoic acid; E carbon dioxide. Please include all steps! Thank you!arrow_forward
- 7. All of the following compounds that are at the same oxidation levelare ___.u. methyl epoxide, v. propyne, w. propanal, x. propene,y. 2,2-dihydroxypropane, z. isopropanol?A. u,v,w,y; B. u,v,w; C. v,w,y,z; D. v, z; E. x,y,z Please include all steps. Thank you!arrow_forward9. Which one of the following substituents is the worst leaving group inan SN2 reaction? A. -NH2; B. -OH; C. –F; D. NH3; E. H2O Please include all steps. Thanks!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 2.5 × 105: CO(g) + H2O(g) CO2(g) + H2(g) Use this information to complete the following table. Suppose a 7.0 L reaction vessel is filled with 1.7 mol of CO and 1.7 mol of H2O. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. CO2(9)+H2(g) CO(g)+H₂O(g) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 CO(g)+3H2O(g) = 3 CO2(g)+3H2(g) There will be very little CO and H2O. x10 There will be very little CO2 and H2. 000 Neither of the above is true. K = ☐ K = ☐ 18 Ararrow_forward
- 8. When ethane thiol is treated with hydrogen peroxide the product is___.A. ethane disulfide; B. diethyl sulfide; C. ethane sulfoxide; D. ethanesulfate; E. ethyl mercaptan. Please include all steps. Thanks!arrow_forward5. The major product of the three step reaction that takes place when 1-propanol is treated with strong acid is?A. dipropyl ether; B. propene; C. propanal; D. isopropyl propyl ether;E. 1-hexanol Please include all steps. Thank you!arrow_forward6. The formula of the product of the addition of HCN to benzaldehydeis ___.A. C8H7NO; B. C8H6NO; C. C14H11NO; D. C9H9NO; E. C9H8NO Please include all steps. Thank you!arrow_forward
- Predicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid K K a name formula name formula nitrous acid HNO2 4.5×10 hydroxylamine HONH2 1.1 × 10 8 hypochlorous acid HCIO 8 3.0 × 10 methylamine CH3NH2 | 4.4 × 10¯ 4 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. 0.1 M KCIO solution PH choose one 0.1 M NaNO2 0.1 M CH3NH3Br 0.1 M NaBr choose one ✓ choose one v ✓ choose one 1 (lowest) ☑ 2 3 4 (highest)arrow_forwardFor this Orgo problem, don't worry about question 3 below it. Please explain your thought process, all your steps, and also include how you would tackle a similar problem. Thank you!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 0.84: H2(g) + 2(g) 2 HI(g) = Use this information to complete the following table. Suppose a 34. L reaction vessel is filled with 0.79 mol of HI. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little H2 and 12. ☐ x10 There will be very little HI. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 HI(g) H₂(9)+12(9) K = What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 H2(g)+212(9) 4 HI(g) K = ☐ ☑arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

