
Concept explainers
(a)
Interpretation:
The electron-pair geometry for the molecules,
Concept introduction:
The electron pairs in Lewis diagrams repel each other in real molecule and thus, they distribute themselves in positions around the central atoms that are as far away from one another. This arrangement of electron pairs is called electron-pair geometry. The electron pairs may be shared in covalent bond, or they may be lone pairs.
Answer to Problem 20E
The Lewis diagrams for
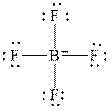 and
and 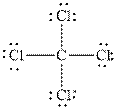
The wedge-and-dash diagrams for
 and
and 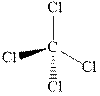
The electron pair geometry for both molecules is tetrahedral.
Explanation of Solution
To write the Lewis diagram for a compound first the number of valence electrons is to be calculated. In the molecule,
Similarly, in the molecule,
The atom which is least electronegative is the central atom. In

Figure 1
In

Figure 2
The electron-pair geometry depends on the number of electron pairs around the central atoms. In both the molecules
The wedge-and-dash diagram for the molecules

Figure 3
The wedge-and-dash diagram for the molecules

Figure 4
The Lewis and wedge-and-dash diagrams for
(b)
Interpretation:
The molecular geometry predicted by the valence shell electron-pair repulsion theory for the molecules
Concept introduction:
Molecular geometry is the precise term that is used to describe the shape of molecules and arrangement of atoms around the central atom. The molecular geometry of a molecule is predicted by valence shell electron-pair repulsion theory or in short VSEPR theory. VSEPR theory applies to substances in which a second period element is bonded to two, three, four, or other atoms.
Answer to Problem 20E
The Lewis diagrams for
 and
and 
The wedge-and-dash diagrams for
 and
and 
The molecular geometry for both molecules is tetrahedral.
Explanation of Solution
To write the Lewis diagram for a compound first the number of valence electrons is to be calculated. In the molecule,
Similarly, in the molecule,
The atom which is least electronegative is the central atom. In

Figure 1
In

Figure 2
The molecular geometry depends on the number of electron pairs as well as number of unpaired electron on the central atoms. In both the molecules
The wedge-and-dash diagram for the molecules

Figure 3
The wedge-and-dash diagram for the molecules

Figure 4
The Lewis and wedge-and-dash diagrams for
Want to see more full solutions like this?
Chapter 13 Solutions
EBK INTRODUCTORY CHEMISTRY: AN ACTIVE L
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





