
College Physics For Ap® Courses
16th Edition
ISBN: 9781938168932
Author: Gregg Wolfe, Irina Lyublinskaya, Douglas Ingram
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 16CQ
What is the vapor pressure of solid carbon dioxide (dry ice) at _785℃
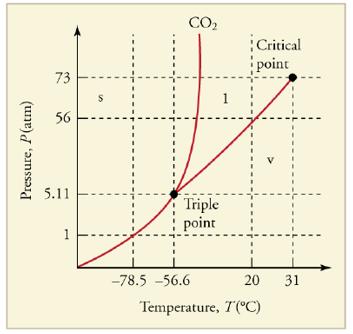
Figure 13.36 The phase diagram for carbon dioxide. The axes aha nonlinear, and the graph is not to scale. Dry ice is solid carbon dioxide and has a sublimation temperature of 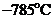
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please solve and answer the question correctly please. Thank you!!
Please solve and answer the question correctly please. Thank you!!
Please solve and answer the questions correctly please. Thank you!!
Chapter 13 Solutions
College Physics For Ap® Courses
Ch. 13 - What does it mean to say that two systems are in...Ch. 13 - Give an example of a physical property that varies...Ch. 13 - When a cold alcohol thermometer is placed in a hot...Ch. 13 - If you add boiling water to a cup at room...Ch. 13 - Thermal stresses caused by uneven cooling can...Ch. 13 - Water expands significantly when it freezes: a...Ch. 13 - One method at getting a tight fit, say of a metal...Ch. 13 - Does it really help to run hot water over a tight...Ch. 13 - Liquids and solids expand with increasing...Ch. 13 - Find out the human population of Earth. Is there a...
Ch. 13 - Under what circumstances would you expect a gas to...Ch. 13 - A constant-volume gas thermometer contains a fixed...Ch. 13 - How is momentum related to the pressure exerted by...Ch. 13 - A pressure cooker contains water and steam in...Ch. 13 - Why does condensation from most rapidly on the...Ch. 13 - What is the vapor pressure of solid carbon dioxide...Ch. 13 - Can carbon dioxide be liquefied at room...Ch. 13 - Oxygen cannot be liquefied at room temperature by...Ch. 13 - What is the distinction between gas and vapor?Ch. 13 - Because humidity depends only on water's vapor...Ch. 13 - Why does a beaker of 40.0C water placed in a...Ch. 13 - Why does rubbing alcohol evaporate much more...Ch. 13 - What is me Fahrenheit temperature of a person with...Ch. 13 - Frost damage to most plants occurs at temperatures...Ch. 13 - To conserve energy, room temperatures are kept at...Ch. 13 - A tungsten light bulb filament may operate a1 2900...Ch. 13 - The Surface temperature of the Sun is about 5750...Ch. 13 - One of the honest temperatures ever recorded on...Ch. 13 - (a) Suppose a cold front blows into your locale...Ch. 13 - (a) At what temperature do the Fahrenheit and...Ch. 13 - The height of the Washington Monument is measured...Ch. 13 - How much taller does me Eiffel Tower become at the...Ch. 13 - What is the change in length of a 3.00mlong column...Ch. 13 - How large an expansion gap should be left between...Ch. 13 - You are looking to purchase a small piece of land...Ch. 13 - Global warming will produce rising sea levels...Ch. 13 - Show that 60.0L of gasoline originally at 15.0C...Ch. 13 - (a) Suppose a meter Stick made of steel and one...Ch. 13 - (a) If a 500mL glass beaker is filled to the brim...Ch. 13 - Most automobiles have a coolant reservoir to catch...Ch. 13 - A physicist makes a cup of instant coffee and...Ch. 13 - (a) The density of water at 0C is very nearly...Ch. 13 - Show that 3, by calculating the change in volume V...Ch. 13 - The gauge pressure in your car tires is...Ch. 13 - Convert an absolute pressure of 7.00105N/m2 to...Ch. 13 - Suppose a gasfilled incandescent light bulb is...Ch. 13 - Large helium-filled balloons are used to lift...Ch. 13 - Confirm mat the units of nRT are those of energy...Ch. 13 - In the text, it was shown that N/V=2.681025m3 for...Ch. 13 - Calculate the number of moles in me 2.00L volume...Ch. 13 - An airplane passenger has 100cm3 of air in his...Ch. 13 - (a) What is me 1imlume (in km3) of Avogadro’s...Ch. 13 - An expensive vacuum System can achieve a pressure...Ch. 13 - The number density of gas atoms at a certain...Ch. 13 - A bicycle tire has a pressure of 7.00105N/m2 at a...Ch. 13 - A high—pressure gas cylinder contains 50.13L of...Ch. 13 - Find the number of moles in 2.00L of gas at 35.0C...Ch. 13 - Calculate the depth to which Avogadro's number of...Ch. 13 - (a) What is me gauge pressure in a 25.0C car tire...Ch. 13 - (a) In the deep space between galaxies, me density...Ch. 13 - Some incandescent light bulbs are filled with...Ch. 13 - Average atomic and molecular speeds (vrms) are...Ch. 13 - (a) What is the average kinetic energy in joules...Ch. 13 - The escape velocity of any object from Earth is...Ch. 13 - The escape velocity from the Moon is much smaller...Ch. 13 - Nuclear fusion, the energy source at the Sun,...Ch. 13 - Suppose that the average velocity (vrms) of carbon...Ch. 13 - Hydrogen molecules (molecular mass is equal to...Ch. 13 - Much of The 935 near the Sun is atomic hydrogen....Ch. 13 - There are two important isotopes of uranium 235U...Ch. 13 - Dry air is 78.1% nitrogen. What is the partial...Ch. 13 - (a) What is me vapor pressure of water at 20.0C ?...Ch. 13 - Pressure cookers increase cooking speed by raising...Ch. 13 - (a) At what temperature does water boil at an...Ch. 13 - What is the atmospheric pressure on top of Mt....Ch. 13 - At a spot in the high Andes, water boils at 80.0C,...Ch. 13 - What is the relative humidity on a 25.0C day when...Ch. 13 - What is the density of water vapor in g/m3 on a...Ch. 13 - A deepsea diver should breathe a gas mixture that...Ch. 13 - The vapor pressure of water at 40.0C is...Ch. 13 - Air in human lungs has a temperature of 37.0C and...Ch. 13 - If the relative humidity is 90.0% on a muggy...Ch. 13 - Late on an autumn day, the relative humidity is...Ch. 13 - Atmospheric pressure amp Mt. Everest is...Ch. 13 - What is the dew point (the temperature at which...Ch. 13 - On a certain day the temperature is 25.0C and the...Ch. 13 - Integrated Concepts The boiling point of water...Ch. 13 - Integrated Concepts (a) At what depth in fresh...Ch. 13 - Integrated Concepts To get an idea of the small...Ch. 13 - Integrated Concepts If you want to cook in water...Ch. 13 - Unreasonable Results (a) How many moles per cubic...Ch. 13 - Unreasonable Results (a) An automobile mechanic...Ch. 13 - Unreasonable Results The temperature inside a...Ch. 13 - Unreasonable Results Suppose the relative humidity...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
Endospore formation is called (a) _____. It is initiated by (b) _____. Formation of a new cell from an endospor...
Microbiology: An Introduction
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
1. A cyclist goes around a level, circular track at constant speed. Do you agree or disagree with the following...
College Physics: A Strategic Approach (3rd Edition)
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
Use a globe or map to determine, as accurately as possible, the latitude and longitude of Athens, Greece.
Applications and Investigations in Earth Science (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- help and explain how to find the answerarrow_forwardWhat is the equivalent resistance between points A and B of the network shown in the figure? A • 12 B 4.0 6.0 Ω 8.0 Ωarrow_forwardAccording to the provided information answer the question accorrding to grade 11 physics Jerry has decided to give up his part-time job for a new career, cat-burglar! Jerry loves the idea of dressing up like a cat all day and of course the chance of meeting Cat Woman! On Jerry's first "job" he figures out his escape plan. He travels 3.0 km south for 15 minutes and then 8.0 km west for 1.5 hours before reaching his house. Draw a sketch diagram of the path he took with all the appropriate labels.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning


Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Kinetic Molecular Theory and its Postulates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=o3f_VJ87Df0;License: Standard YouTube License, CC-BY