
(a)
Interpretation:
The bubble point pressure for any one of the given binary systems in table
Concept Introduction:
Antoine equation is used to determine the vapor pressure of any substance at the given temperature by the equation:
Here,
Equation
The Bubble point pressure for a binary system in vapor/liquid equilibrium is defined as the pressure where first bubble of vapor appears which is in equilibrium with the liquid present in the system. The equation which defines this pressure at this point is:
NRTL equations to be used are:
Here, the parameters
And,
Where,
(a)
Answer to Problem 13.48P
The bubble point pressure for
Explanation of Solution
Given information:
The temperature at which the bubble point pressure is to be calculated is
NRTL equation parameters are given in Table 13.10 as shown below:
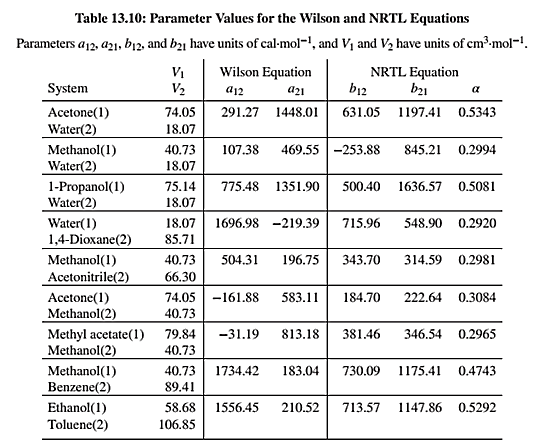
The binary system for which the bubble point pressure will be calculated is
From table B.2 of appendix B, the Antoine equation constants for
Now, use equation (1) to calculate the vapor pressure of
From table
The value of universal gas constant to be used is,
Now, use equation (5) to calculate the values of
Use equation (6) to calculate the values of
Now, use these values of
Calculate the bubble point pressure of the system using equation (3) as:
(b)
Interpretation:
The dew point pressure for any one of the given binary systems in table
Concept Introduction:
Equation
NRTL equations to be used are:
Here, the parameters
And,
Where,
The Dew point pressure for a binary system in vapor/liquid equilibrium is defined as the pressure where first drop of liquid appears which is in equilibrium with the vapor present in the system at a particular temperature. The equation that defines this pressure at this point is:
(b)
Answer to Problem 13.48P
The dew point pressure for
Explanation of Solution
Given information:
The temperature at which the dew point pressure is to be calculated is
NRTL equation parameters are given in Table 13.10 as shown below:
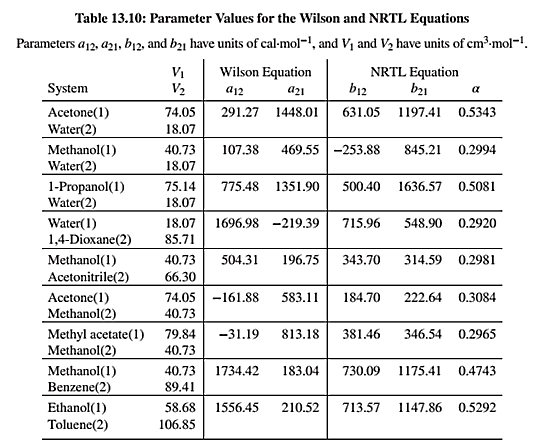
Use the values of
From table
The value of universal gas constant to be used is,
Now, use equation (5) to calculate the values of
Use equation (6) to calculate the values of
1st iteration:
Now, use these values of
Now, calculate the dew point pressure of the system using equation (7) as:
Apply Raoult’s law on both the components and use this dew point pressure to calculate
Now, use this calculated value of
2nd iteration:
Calculate the values of
Now, calculate the dew point pressure of the system using equation (7) as:
Apply Raoult’s law on both the components and use this dew point pressure to calculate
3rd iteration:
Calculate the values of
Now, calculate the dew point pressure of the system using equation (7) as:
Apply Raoult’s law on both the components and use this dew point pressure to calculate
4th iteration:
Calculate the values of
Now, calculate the dew point pressure of the system using equation (7) as:
Apply Raoult’s law on both the components and use this dew point pressure to calculate
5th iteration:
Calculate the values of
Now, calculate the dew point pressure of the system using equation (7) as:
Apply Raoult’s law on both the components and use this dew point pressure to calculate
Since,
Therefore,
(c)
Interpretation:
Concept Introduction:
Equation
The Bubble point pressure for a binary system in vapor/liquid equilibrium is defined as the pressure where first bubble of vapor appears which is in equilibrium with the liquid present in the system. The equation which defines this pressure at this point is:
The Dew point pressure for a binary system in vapor/liquid equilibrium is defined as the pressure where first drop of liquid appears which is in equilibrium with the vapor present in the system at a particular temperature. The equation that defines this pressure at this point is:
The equation for equilibrium ratio,
Here,
The equations for flash calculations to be used are:
Here,
In terms of
Here,
(c)
Answer to Problem 13.48P
The result of the
Explanation of Solution
Given information:
The flash temperature at which the
The condition for the flash pressure for this system is,
NRTL equation parameters are given in Table 13.10 as shown below:
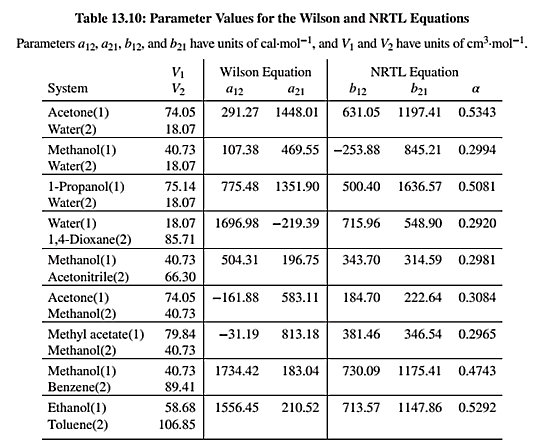
Use the values of
To perform
To calculate bubble point pressure, let
Since, the given conditions are same as in part (a), the calculated value of
To calculate dew point pressure, let
Since, the given conditions are same as in part (b), the calculated value of
From the given condition of the flash pressure, it is calculated as:
Now, using the modified Raoult’s law, calculate the values of equilibrium ratio of component 1 and 2 using equations (2) and (8) as:
Now, use equation (10) and write it for both the component, 1 and 2 as shown below:
Since,
Now, use equation (9) to calculate the value of
Also, use the calculated value of
Using these values and the calculated values of
The result of the
(d)
Interpretation:
The values of the azeotropic pressure and composition of the system is to be calculated if it exists for the given binary system.
Concept Introduction:
Equation
NRTL equations to be used are:
Here, the parameters
And,
Where,
Relative volatility is defined by,
When
At the azeotropic point,
(d)
Answer to Problem 13.48P
The azeotropic values of pressure and composition for the binary system is calculated as:
Explanation of Solution
Given information:
The temperature at which the azeotrope of the system may exists is
NRTL equation parameters are given in Table 13.10 as shown below:
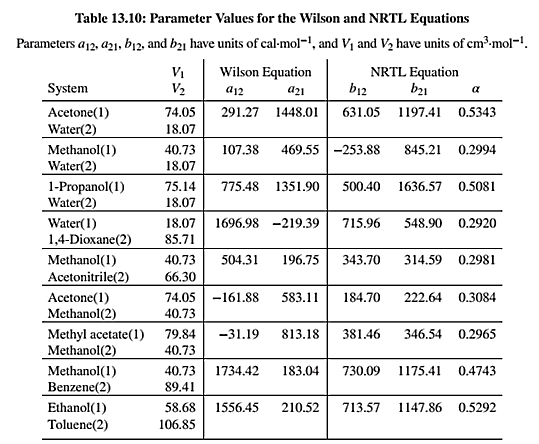
Use the values of
From table
The value of universal gas constant to be used is,
Now, use equation (5) to calculate the values of
Use equation (6) to calculate the values of
Now, use these values of
Using equation (12) along with the modified Raoult’s law, calculate the value of relative volatility at
For
Using equation (12) along with the modified Raoult’s law, calculate the value of relative volatility at
Since
To calculate the azeotropic pressure, consider the condition
1st iteration:
Now, use the values of
Now, calculate the azeotropic pressure of the system as:
Apply Raoult’s law on both the components and use this pressure to calculate
Now, use this calculated value of
Want to see more full solutions like this?
Chapter 13 Solutions
INTRO.TO CHEM.ENGR.THERMO.-EBOOK>I<
- Exhaust gas from a power plant passes through a 15-by-20-it rectangular duct at an average velocity of 50 ft/s. The total length of duct is 250 ft and there are two 90° bends.The gas is at 180°F and about 1 atm, and the properties are similar to those of air. Calculate the pressure drop in the duet and the power required to overcome pressure losses.arrow_forwardUntuk sistem gas etilena (1)/propilena (2), estimasi (f^1, f^2, $^1, dan ^2 pada t = 150°C, P = 30 bar, dan y1 = 0,35; kij = 0. (a) Dengan menerapkan Persamaan (10.63). (b) Dengan asumsi bahwa campuran adalah lingkungan idealarrow_forwardOnly focus on H(3), which is the specific enthalpy for nitrogen gas. chemical engineeringarrow_forward
- chemical engineering. Only focus on H(3), which is the nitrogen gas. Start with the reference state to the process state. Be thorough to the fullestarrow_forwardacetone with these parameters: po:=101325; #Standard atmospheric pressure in PaTfo:=273.15-94.45; #Melting temperature in K Tvo:=273.15+56.15; #Boiling temperature in K Hv:=31270; #Enthalpy of vaporization in J/molR:=8.314; #Gas Constant in J/mol*KNLe:=1.76; #Lewis number for acetoneMw:= 0.05808 ; #kg/mol molecular weight of acetoneW0:= 0.15; Wsp:=0.005;Am:= 0.12; #m^2/kg dry solid for the exposed wet areah:= 11; #W/m^2K for heat transfer coefficienttau__min:= Hv*(W0-Wsp)/Mw/Am/h/(T8-TS); tau__min/60;arrow_forwardchemical engineering Material-energy balance. Only focus on the nitrogen gas, which is H(3)arrow_forward
- 1. The settling chamber, shown schematically in Figure 2E1.1, is used as a primary separation device in the removal of dust particles of density 1500 kg/m³ from a gas of density 0:7 kg/m³ and viscosity 1.90 x 10-5 Pa s. Gas inlet Elevation Gas Gas exit exit H Collection surface -W Section X-X Dimensions: H=3m L = 10 m W=2m Figure 2E1.1 Schematic diagram of settling chamber Assuming Stokes' law applies, show that the efficiency of collection of particles of size x is given by the expression collection efficiency, x = x²8(pp - Pi)L 18μHU where U is the uniform gas velocity through the parallel-sided section of the chamber. State any other assumptions made. (b) What is the upper limit of particle size for which Stokes' law applies? (c) When the volumetric flow rate of gas is 0.9 m³/s, and the dimensions of the chamber are those shown in Figure 2E1.1, determine the collection efficiency for spherical particles of diameter 30 mm.arrow_forwardCan you answer this sequantially correct like show me the full process. Also, since it is chemical engineering related problem a perry's handbook is used. Thank youarrow_forwardchemical engineering Demonstrate how each specific enthalpy was calculated, from the reference state to the process state. Be thorough to the fullest. This is a material-energy balance. The answers are H(1) = 35.7 KJ/kmol, H(2) = 32.0 KJ/kmol, and H(3) = -1.26 KJ/kmol.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





