
Concept explainers
(a)
Interpretation:
An
Concept introduction:
E2 stands for bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the
Answer to Problem 13.35P
An alkyl halide that could have been used to synthesize the known alkene exclusively via an E2 reaction paying attention to stereochemistry is:
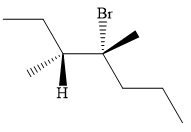
Explanation of Solution
The structure of the desired alkene is:
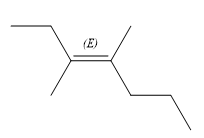
The given alkene has both the higher priority groups attached on the opposite side of the double bond. Hence, the stereochemistry is E. Alkenes can be prepared from corresponding alkyl halides via E2 reactions. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to prepare the given alkene must be:

To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(b)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
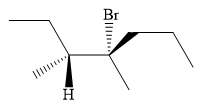
Explanation of Solution
The structure of the required alkene is:
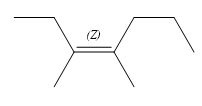
The given alkene has both the most priority groups attached on the same side of the double bond. Therefore, the stereochemistry about the double bond is Z. Alkenes can be prepared from corresponding alkyl halides via E2 reactions. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to prepare the given alkene must be:

To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(c)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
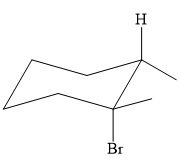
Explanation of Solution
The structure of the desired alkene is:
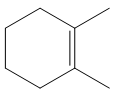
The alkene is a cycloalkene having two methyl groups attached to the double-bonded carbon atoms. As the carbon atoms in alkenes are

To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(d)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
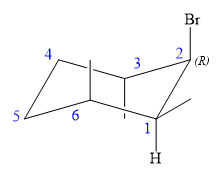
Explanation of Solution
The structure of the desired alkene is:
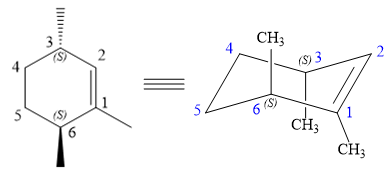
The alkene is cyclohexene having three methyl groups as substituents.
As the carbon atoms in alkenes are sp2 hybridized, all the atoms that are directly attached to the double-bonded carbon atoms must be in the plane. There are two chiral centers in the molecule at C3 and C6 carbon atoms. The stereochemistry for those two chiral centers will not change and will be retained as the reaction does not occur at those two chiral centers. Alkenes can be prepared from corresponding alkyl halides via E2 reactions.
This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to prepare the given alkene must be:
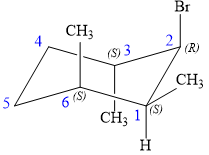
To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
(e)
Interpretation:
An alkyl halide, that can be used to synthesize the given alkene exclusively via an E2 reaction paying attention to stereochemistry, is to be provided.
Concept introduction:
The name E2 represents bimolecular elimination. This reaction is a one-step concerted mechanism. In this step, the C-X and C-H bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Since the alkyl halide and base influence the rate of reaction, this is a bimolecular reaction. A strong base is used to form the most substituted alkene as the major product.
Answer to Problem 13.35P
An alkyl halide that could have been used to prepare the given alkene exclusively via an E2 reaction paying attention to stereochemistry is:
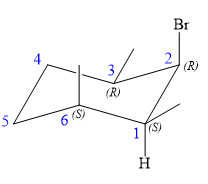
Explanation of Solution
The structure of the desired alkene is:
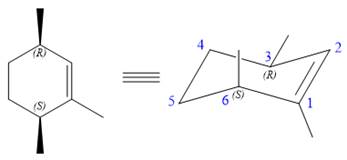
The alkene is cyclohexene having three methyl groups as substituents.
As the carbon atoms in alkenes are sp2 hybridized, all the atoms that are directly attached to the double-bonded carbon atoms must be in the plane. There are two chiral centers in the molecule at C3 and C6 carbon atoms. The stereochemistry for those two chiral centers will not change and will be retained as the reaction does not occur at those two chiral centers. Alkenes can be prepared from corresponding alkyl halides via E2 reactions.
This reaction involves a one-step mechanism (concerted) in which carbon-halogen bond and carbon-hydrogen bond breaks to form a double bond. The leaving group and the adjacent hydrogen atom must be anticoplanar in the precursor for the E2 step to be favored. To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion. Thus, the original alkyl halide that could have been used to synthesize the given alkene must be:
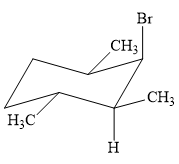
To arrive back at the alkyl halide, one must add hydrogen and a halogen on the alkene carbons in an anti-fashion.
Want to see more full solutions like this?
Chapter 13 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- One method for the analysis of Fe3+, which is used with a variety of sample matrices, is to form the highly colored Fe3+–thioglycolic acid complex. The complex absorbs strongly at 535 nm. Standardizing the method is accomplished using external standards. A 10.00-ppm Fe3+ working standard is prepared by transferring a 10-mL aliquot of a 100.0 ppm stock solution of Fe3+ to a 100-mL volumetric flask and diluting to volume. Calibration standards of 1.00, 2.00, 3.00, 4.00, and 5.00 ppm are prepared by transferring appropriate amounts of the 10.0 ppm working solution into separate 50-mL volumetric flasks, each of which contains 5 mL of thioglycolic acid, 2 mL of 20% w/v ammonium citrate, and 5 mL of 0.22 M NH3. After diluting to volume and mixing, the absorbances of the external standards are measured against an appropriate blank. Samples are prepared for analysis by taking a portion known to contain approximately 0.1 g of Fe3+, dissolving it in a minimum amount of HNO3, and diluting to…arrow_forwardAbsorbance and transmittance are related by: A = -log(T) A solution has a transmittance of 35% in a 1-cm-pathlength cell at a certain wavelength. Calculate the transmittance if you dilute 25.0 mL of the solution to 50.0 mL? (A = εbc) What is the transmittance of the original solution if the pathlength is increased to 10 cm?arrow_forwardUnder what conditions will Beer’s Law most likely NO LONGER be linear? When the absorbing species is very dilute. When the absorbing species participates in a concentration-dependent equilibrium. When the solution being studied contains a mixture of ions.arrow_forward
- Compared to incident (exciting) radiation, fluorescence emission will have a: Higher energy Higher frequency Longer wavelengtharrow_forwardLin and Brown described a quantitative method for methanol based on its effect on the visible spectrum of methylene blue. In the absence of methanol, methylene blue has two prominent absorption bands at 610 nm and 663 nm, which correspond to the monomer and the dimer, respectively. In the presence of methanol, the intensity of the dimer’s absorption band decreases, while that for the monomer increases. For concentrations of methanol between 0 and 30% v/v, the ratio of the two absorbance, A663/ A610, is a linear function of the amount of methanol. Use the following standardization data to determine the %v/v methanol in a sample if A610 is 0.75 and A663 is 1.07.arrow_forwardThe crystal field splitting energy, Δ, of a complex is determined to be 2.9 × 10-19 What wavelength of light would this complex absorb? What color of light is this? What color would the compound be in solution?arrow_forward
- A key component of a monochromator is the exit slit. As the exit slit is narrowed, the bandwidth of light (i.e., the range of wavelengths) exiting the slit gets smaller, leading to higher resolution. What is a possible disadvantage of narrowing the exit slit? (Hint: why might a narrower slit lower the sensitivity of the measurement?).arrow_forwardAn x-ray has a frequency of 3.33 × 1018 What is the wavelength of this light?arrow_forwardChoose the Lewis structure for the compound below: H2CCHOCH2CH(CH3)2 HH H :d H H H C. Η H H HH H H H H. H H H HH H H H H H- H H H C-H H H HHHHarrow_forward
- Each of the highlighted carbon atoms is connected to hydrogen atoms.arrow_forwardく Complete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. Explanation Check O + G 1. Na O Me Click and drag to start drawing a structure. 2. H + 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 000 Ar Parrow_forwardDraw a tetramer of this alternating copolymer.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

