
(a)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to the product by reacting with reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on the right side of the reaction arrow
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on the right side of the reaction arrow
(![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
The starting ![]() . Then, treat
. Then, treat ![]() with phosphoric acid at
with phosphoric acid at ![]() to form
to form ![]() .
.
Explanation of Solution
The given synthesis scheme is:
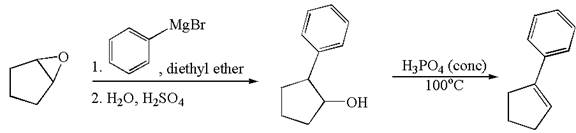
In the given synthetic route, the reactant having functional group epoxide is converted to an alcohol named ![]() which is further converted to an
which is further converted to an ![]() on reaction with appropriate reagents as mentioned. In the first step, the first reagent used is phenylmagnesium bromide in the solvent diethyl ether, and the second reagent is
on reaction with appropriate reagents as mentioned. In the first step, the first reagent used is phenylmagnesium bromide in the solvent diethyl ether, and the second reagent is ![]() which represents the aqueous acidic condition. In the second step, the reagent is phosphoric acid and
which represents the aqueous acidic condition. In the second step, the reagent is phosphoric acid and ![]() is the reaction temperature. Thus, the word form of the above synthetic scheme can be written as follows:
is the reaction temperature. Thus, the word form of the above synthetic scheme can be written as follows:
The starting epoxide reacts with phenylmagnesium bromide in the solvent diethyl ether followed by aqueous acid, to form ![]() . Then, treat
. Then, treat ![]() with phosphoric acid at
with phosphoric acid at ![]() to form
to form ![]() .
.
The given synthesis scheme is converted to word form by identifying the names of reactants, reagents, and products.
(b)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to products by reacting with the reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants and the functional groups produced in the product are to be identified.
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants and the functional groups produced in the product are to be identified.
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
Phenylehtanone reacts with lithium diisopropylamide followed by iodomethane, to produce phenylpropanone. Then, add the molecular bromine in the presence of acetic acid to form ![]() which further reacts with sodium acetate to yield the final product.
which further reacts with sodium acetate to yield the final product.
Explanation of Solution
The given synthesis scheme is:
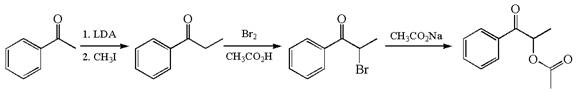
The given synthetic route is of three steps. In the first step, the reactant having functional group ![]() alpha bromo ketone named
alpha bromo ketone named ![]() , which, in the third step, is further converted to the final product having the ester functional group. In the first step, the first reagent used is lithium diisopropylamide, and the second reagent is iodomethane. In the second step, the reagent is molecular bromine in acetic acid. The reagent for the third step is sodium acetate. Thus, the word form of the above synthetic scheme can be written as follows:
, which, in the third step, is further converted to the final product having the ester functional group. In the first step, the first reagent used is lithium diisopropylamide, and the second reagent is iodomethane. In the second step, the reagent is molecular bromine in acetic acid. The reagent for the third step is sodium acetate. Thus, the word form of the above synthetic scheme can be written as follows:
Phenylehtanone reacts with lithium diisopropylamide followed by iodomethane to produce phenylpropanone. Add the molecular bromine in presence of acetic acid to phenylpropanone to form ![]() which further reacts with sodium acetate to yield THE final product.
which further reacts with sodium acetate to yield THE final product.
The given synthesis scheme was converted to word form by identifying the names of reactants, reagents, and products.
(c)
Interpretation:
The given synthesis scheme is to be converted to word form that can be used as instructions in the laboratory.
Concept introduction:
The synthesis scheme is the balanced chemical equation written for carrying out a sequence of reactions with specified steps. The synthetic step displays that the reactants are converted to the product by reacting with reagents in the required conditions.
The structures on the left side of the reaction arrow (![]() ) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (
) are the reactants. The reagents used are written above the arrow, and the reaction conditions including solvent, temperature, pH, time of reaction, etc. are written below the arrow. If more than one sequence is combined in one step, then the reagents are numbered according to their sequence and can be written above and below the reaction arrow. In such cases, the reaction conditions are separated from the reactant or reagent by either a comma or a slash. The numbers given to reagents represent that the reaction goes to completion before the next reagent is added. The structures on right side of the reaction arrow (![]() ) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants, and the functional groups produced in the product are to be identified.
) are the products. The product of the previous step is the reactant of the next step. While writing the synthetic scheme in word form, the functional groups involved in the reactants, and the functional groups produced in the product are to be identified.
Answer to Problem 13.28P
The word form for the given synthesis scheme is:
Heat ![]() in aqueous hydrochloric acid to form
in aqueous hydrochloric acid to form ![]() , add phosphorous tribromide to it to produce
, add phosphorous tribromide to it to produce ![]() . Then, react
. Then, react ![]() with sodium azide to yield the final product.
with sodium azide to yield the final product.
Explanation of Solution
The given synthesis scheme is:
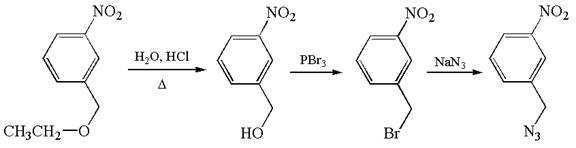
The given synthetic route is of three steps. The nitro functional group remains as it is throughout the reaction sequence, and so, is not considered. In the first step, ether functional group is converted to an alcohol. In the second step, ![]() group of alcohol is replaced by bromine, which in the third step is replaced by
group of alcohol is replaced by bromine, which in the third step is replaced by ![]() . In the first step, the reagent used is
. In the first step, the reagent used is ![]() that represents acidic condition where
that represents acidic condition where ![]() is the symbol used for heat. In the second step, the reagent is
is the symbol used for heat. In the second step, the reagent is ![]() , phosphorous tribromide, and in the third step, the reagent used is sodium azide,
, phosphorous tribromide, and in the third step, the reagent used is sodium azide, ![]() . Thus, the word form of the above synthetic scheme can be written as follows:
. Thus, the word form of the above synthetic scheme can be written as follows:
Heat ![]() in aqueous hydrochloric acid to form
in aqueous hydrochloric acid to form ![]() , and add phosphorous tribromide to it to produce
, and add phosphorous tribromide to it to produce ![]() . Then, react
. Then, react ![]() with sodium azide to yield the final product.
with sodium azide to yield the final product.
The given synthesis scheme was converted to word form by identifying the names of reactants, reagents, and products.
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry: Principles And Mechanisms
- CUE COLUMN NOTES (A. Determine Stereoisomers it has ⑤ Identify any meso B compounds cl Br cl -c-c-c-c-¿- 1 CI C- | 2,4-Dichloro-3-bromopentanearrow_forwardThe acid-base chemistry of both EDTA and EBT are important to ensuring that the reactions proceed as desired, thus the pH is controlled using a buffer. What percent of the EBT indicator will be in the desired HIn2- state at pH = 10.5. pKa1 = 6.2 and pKa2 = 11.6 of EBTarrow_forwardWhat does the phrase 'fit for purpose' mean in relation to analytical chemistry? Please provide examples too.arrow_forward
- For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects Resonance Effects Overall Electron-Density × NO2 ○ donating O donating O withdrawing O withdrawing O electron-rich electron-deficient no inductive effects O no resonance effects O similar to benzene E [ CI O donating withdrawing O no inductive effects Explanation Check ○ donating withdrawing no resonance effects electron-rich electron-deficient O similar to benzene © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardUnderstanding how substituents activate Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation HN NH2 Check X (Choose one) (Choose one) (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Aarrow_forwardIdentifying electron-donating and electron-withdrawing effects on benzene For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Inductive Effects Resonance Effects Overall Electron-Density Molecule CF3 O donating O donating O withdrawing O withdrawing O no inductive effects O no resonance effects electron-rich electron-deficient O similar to benzene CH3 O donating O withdrawing O no inductive effects O donating O withdrawing Ono resonance effects O electron-rich O electron-deficient O similar to benzene Explanation Check Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- * Hint: Think back to Chem 1 solubility rules. Follow Up Questions for Part B 12. What impact do the following disturbances to a system at equilibrium have on k, the rate constant for the forward reaction? Explain. (4 pts) a) Changing the concentration of a reactant or product. (2 pts) b) Changing the temperature of an exothermic reaction. (2 pts) ofarrow_forwardDraw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forwardPlease help me with the following questions for chemistry.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


