
Kinetics I
Consider the hypothetical reaction A(g) + 2B(g) h C(g). The four containers below represent this reaction being run with different initial amounts of A and B. Assume that the volume of each container is 1.0 L. The reaction is second order with respect to A and first order with respect to B.
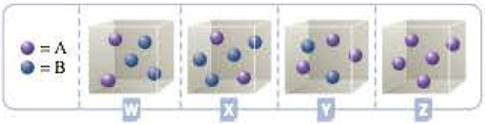
- a Based on the information presented in the problem, write the rate law for the reaction.
- b Which of the containers, W, X, Y, or Z, would have the greatest reaction rate? Justify your answer.
- c Which of the containers would have the lowest reaction rate? Explain.
- d If the volume of the container X were increased to 2.0 L, how would the rate of the reaction in this larger container compare to the
rate of reaction run in the 1.0-L container X? (Assume that the number of A and B atoms is the same in each case.) - e If the temperature in container W were increased, what impact would this probably have on the rate of reaction? Why?
- f If you want to double the rate of reaction in container X, what are some things that you could do to the concentration(s) of A and B?
- g In which container would you observe the slowest rate of formation of C?
- h Assuming that A and B are not in great excess, which would have the greater impact on the rate of reaction in container W: removing a unit of B or removing a unit of A? Explain.
- i Describe how the rate of consumption of A compares to the rate of consumption of B. If you cannot answer this question, what additional information do you need to provide an answer?
- j If the product C were removed from the container as it formed, what effect would this have on the rate of the reaction?
(a)
Interpretation:
The explanations for the given set of statements have to be given.
Concept Introduction:
The rate of reaction is the quantity of formation of product or the quantity of reactant used per unit time. The rate of reaction doesn’t depend on the sum of amount of reaction mixture used.
The raise in molar concentration of product of a reaction per unit time or decrease in molarity of reactant per unit time is called rate of reaction and is expressed in units of
The variation in concentration of reaction or product over a certain interval of time is called average reaction rate.
The equation that relates the reaction rate to the reactants concentrations that is raised to various powers is called as rate law.
Rate law can be determined by the slow step or otherwise called as rate-determining step.
Explanation of Solution
To give rate of the reaction
Rate of the reaction is given as
(b)
Interpretation:
The explanations for the given set of statements have to be given.
Concept Introduction:
The rate of reaction is the quantity of formation of product or the quantity of reactant used per unit time. The rate of reaction doesn’t depend on the sum of amount of reaction mixture used.
The raise in molar concentration of product of a reaction per unit time or decrease in molarity of reactant per unit time is called rate of reaction and is expressed in units of
The variation in concentration of reaction or product over a certain interval of time is called average reaction rate.
The equation that relates the reaction rate to the reactants concentrations that is raised to various powers is called as rate law.
Rate law can be determined by the slow step or otherwise called as rate-determining step.
Explanation of Solution
To identify and justify the container that has greatest rate of reaction
The container with the highest rate of reaction will have highest value of
For container W, the product will be
For container X, the product will be
For container Y, the product will be
For container Z, the product will be
Hence, the container Y will have the highest rate of reaction.
(c)
Interpretation:
The explanations for the given set of statements have to be given.
Concept Introduction:
The rate of reaction is the quantity of formation of product or the quantity of reactant used per unit time. The rate of reaction doesn’t depend on the sum of amount of reaction mixture used.
The raise in molar concentration of product of a reaction per unit time or decrease in molarity of reactant per unit time is called rate of reaction and is expressed in units of
The variation in concentration of reaction or product over a certain interval of time is called average reaction rate.
The equation that relates the reaction rate to the reactants concentrations that is raised to various powers is called as rate law.
Rate law can be determined by the slow step or otherwise called as rate-determining step.
Explanation of Solution
To identify and justify the container that has lowest rate of reaction
Since the container Z has one of the concentrations of reactant as zero, container Z will have the lowest rate of reaction.
(d)
Interpretation:
The explanations for the given set of statements have to be given.
Concept Introduction:
The rate of reaction is the quantity of formation of product or the quantity of reactant used per unit time. The rate of reaction doesn’t depend on the sum of amount of reaction mixture used.
The raise in molar concentration of product of a reaction per unit time or decrease in molarity of reactant per unit time is called rate of reaction and is expressed in units of
The variation in concentration of reaction or product over a certain interval of time is called average reaction rate.
The equation that relates the reaction rate to the reactants concentrations that is raised to various powers is called as rate law.
Rate law can be determined by the slow step or otherwise called as rate-determining step.
Explanation of Solution
To compare the rate of reaction in the larger container to the rate of reaction in
The concentrations of A and B are decreased by factor 2, when the volume of the container is two times from
Therefore, the rate of reaction in larger container is
(e)
Interpretation:
The explanations for the given set of statements have to be given.
Concept Introduction:
The rate of reaction is the quantity of formation of product or the quantity of reactant used per unit time. The rate of reaction doesn’t depend on the sum of amount of reaction mixture used.
The raise in molar concentration of product of a reaction per unit time or decrease in molarity of reactant per unit time is called rate of reaction and is expressed in units of
The variation in concentration of reaction or product over a certain interval of time is called average reaction rate.
The equation that relates the reaction rate to the reactants concentrations that is raised to various powers is called as rate law.
Rate law can be determined by the slow step or otherwise called as rate-determining step.
Explanation of Solution
To give the impact on the rate of reaction if temperature is increased in container W
Increase in temperature, increases the rate of temperature,
At higher temperature molecules collide with other molecules at greater rate and possess greater kinetic energy.
Thus, part of collision with energy in addition of activation energy is greater with increase in rate of reaction.
(f)
Interpretation:
The explanations for the given set of statements have to be given.
Concept Introduction:
The rate of reaction is the quantity of formation of product or the quantity of reactant used per unit time. The rate of reaction doesn’t depend on the sum of amount of reaction mixture used.
The raise in molar concentration of product of a reaction per unit time or decrease in molarity of reactant per unit time is called rate of reaction and is expressed in units of
The variation in concentration of reaction or product over a certain interval of time is called average reaction rate.
The equation that relates the reaction rate to the reactants concentrations that is raised to various powers is called as rate law.
Rate law can be determined by the slow step or otherwise called as rate-determining step.
Explanation of Solution
To explain about the concentrations of
The rate of the reactions doubles with rate of products
(g)
Interpretation:
The explanations for the given set of statements have to be given.
Concept Introduction:
The rate of reaction is the quantity of formation of product or the quantity of reactant used per unit time. The rate of reaction doesn’t depend on the sum of amount of reaction mixture used.
The raise in molar concentration of product of a reaction per unit time or decrease in molarity of reactant per unit time is called rate of reaction and is expressed in units of
The variation in concentration of reaction or product over a certain interval of time is called average reaction rate.
The equation that relates the reaction rate to the reactants concentrations that is raised to various powers is called as rate law.
Rate law can be determined by the slow step or otherwise called as rate-determining step.
Explanation of Solution
To identify the container that shows slowest rate for the formation of C
Container Z shows the slowest rate for the formation of C because its reaction rate is zero.
(h)
Interpretation:
The explanations for the given set of statements have to be given.
Concept Introduction:
The rate of reaction is the quantity of formation of product or the quantity of reactant used per unit time. The rate of reaction doesn’t depend on the sum of amount of reaction mixture used.
The raise in molar concentration of product of a reaction per unit time or decrease in molarity of reactant per unit time is called rate of reaction and is expressed in units of
The variation in concentration of reaction or product over a certain interval of time is called average reaction rate.
The equation that relates the reaction rate to the reactants concentrations that is raised to various powers is called as rate law.
Rate law can be determined by the slow step or otherwise called as rate-determining step.
Explanation of Solution
To identify and explain if removing A or B would have higher impact on rate of reaction of container W
Reaction in A is second order.
Reaction in B is first order.
Changes in concentration of A would have higher impact on rate of reaction; hence removing A would have higher impact on rate of reaction of container W
(i)
Interpretation:
The explanations for the given set of statements have to be given.
Concept Introduction:
The rate of reaction is the quantity of formation of product or the quantity of reactant used per unit time. The rate of reaction doesn’t depend on the sum of amount of reaction mixture used.
The raise in molar concentration of product of a reaction per unit time or decrease in molarity of reactant per unit time is called rate of reaction and is expressed in units of
The variation in concentration of reaction or product over a certain interval of time is called average reaction rate.
The equation that relates the reaction rate to the reactants concentrations that is raised to various powers is called as rate law.
Rate law can be determined by the slow step or otherwise called as rate-determining step.
Explanation of Solution
To compare the rate of consumptions of A and B
The rate of reactions is,
The rate of consumption of A is half the rate of consumption of B.
(j)
Interpretation:
The explanations for the given set of statements have to be given.
Concept Introduction:
The rate of reaction is the quantity of formation of product or the quantity of reactant used per unit time. The rate of reaction doesn’t depend on the sum of amount of reaction mixture used.
The raise in molar concentration of product of a reaction per unit time or decrease in molarity of reactant per unit time is called rate of reaction and is expressed in units of
The variation in concentration of reaction or product over a certain interval of time is called average reaction rate.
The equation that relates the reaction rate to the reactants concentrations that is raised to various powers is called as rate law.
Rate law can be determined by the slow step or otherwise called as rate-determining step.
Explanation of Solution
To give the rate of reaction if product is taken away from the container
Removing C from the container shoes no effect on the reaction rate because
Want to see more full solutions like this?
Chapter 13 Solutions
Student Solutions Manual for Ebbing/Gammon's General Chemistry, 11th
- Please label this COZY spectraarrow_forwardPlease label this HNMRarrow_forwardConsider the following gas chromatographs of Compound A, Compound B, and a mixture of Compounds A and B. Inject A B mixture Area= 9 Area = 5 Area = 3 Area Inject . མི། Inject J2 What is the percentage of Compound B in the the mixture?arrow_forward
- Rank these according to stability. CH3 H3C CH3 1 CH3 H3C 1 most stable, 3 least stable O 1 most stable, 2 least stable 2 most stable, 1 least stable O2 most stable, 3 least stable O3 most stable, 2 least stable O3 most stable, 1 least stable CH3 2 CH3 CH3 H₂C CH3 3 CH3 CHarrow_forwardConsider this IR and NMR: INFRARED SPECTRUM TRANSMITTANCE 0.8- 0.6 0.4 0.2 3000 10 9 8 00 HSP-00-541 7 CO 6 2000 Wavenumber (cm-1) сл 5 ppm 4 M Which compound gave rise to these spectra? N 1000 1 0arrow_forwardConsider this reaction (molecular weights are under each compound): HC=CH + 2 HCI --> C2H4Cl 2 MW = 26 36.5 99 If 4.4 g of HC=CH are reacted with 110 mL of a 2.3 M HCI solution, and 6.0 g of product are actually produced, what is the percent yield?arrow_forward
- What is the name of the major product of this reaction? OH CH3 H₂SO4, heat 1-methylcyclohexene O2-methyl-1-cyclohexene O 3-mthylcyclohexene 1-methyl-2-cyclohexenearrow_forwardWe added a brown solution of Br2 to one of our products, and the brown color disappeared. This indicated that our product wasarrow_forwardRank the following according to reactivity toward nitration: a) benzene b) bromobenzene c) nitrobenzene d) phenol Od) greatest, c) least Od) greatest, b) least Od) greatest, a) least a) greatest, b) least a) greatest, c) least Oa) greatest, d) least Ob) greatest, a) least O b) greatest, c) least Ob) greatest, d) least O c) greatest, a) least O c) greatest, b) least O c) greatest, d) leastarrow_forward
- O-Nitrophenol was distilled over with the steam in our experiment while the other isomer did not. This is due to: O intramolecular hydrogen bonding in the ortho isomer O intermolecular hydrogen bonding in the the ortho isomer O the ortho isomer has a lower density O the ortho isomer has a lower molecular weightarrow_forwardK 44% Problem 68 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :6: :: :CI: CI CI: :0:0 Select to Add Arrows Select to Add Arrows H H Cl CI: CI CI: Select to Add Arrows Select to Add Arrows H :CI: Alarrow_forwardI I H :0: Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. 0:0 :0: CI ΑΙ :CI: :CI: :0: CI Select to Add Arrows Select to Add Arrows cl. :0: Cl © ハ CI:: CI H CO Select to Add Arrows Select to Add Arrows 10: AI ::arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





