
(a)
Interpretation:
From mass balance 13.15, the equation 13-16 has to be derived.
Concept Introduction:
Ion-pairing in acid-base systems:
The possible ion-pair equilibria in the mixture of sodium hydrogen tartrate
(a)
Explanation of Solution
Derivation of equation 13-16 from mass balance 13-15:
Equation 13-15 (mass balance for sodium) is:
Equation 13-16 is:
Consider the two equations,
Substitute
Hence, the equation 13-16 is derived from mass balance 13.15.
(b)
Interpretation:
An expression for
Concept Introduction:
Mass balance equation for
The mass balance equation for
(b)
Answer to Problem 13.17P
The expression for
Explanation of Solution
Make the following substitutions to find expressions in terms of
Put these expressions into the mass balance equation:
Solve for
Hence, the expression for
The expression for
(c)
Interpretation:
Using the same approach in part (b), the expression for
Concept Introduction:
Mass balance equation for
The mass balance equation for
(c)
Answer to Problem 13.17P
The expression for
Explanation of Solution
To find
And now solve for
To find
Then keep these expressions into the mass balance:
And solve for
The expression for
(d)
Interpretation:
Using excel spreadsheet, the pH of the given solution has to be computed.
(d)
Answer to Problem 13.17P
The pH of the given solution is
Explanation of Solution
The spreadsheet used to compute pH of the given solution is shown in the below figure 1.
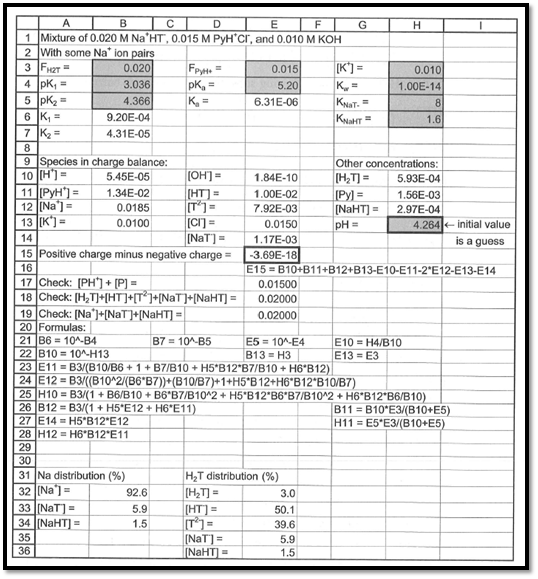
Figure 1
From the above spreadsheet, the pH of the solution is found as
The pH of the given solution is found out as
Want to see more full solutions like this?
Chapter 13 Solutions
Solution Manual for Quantitative Chemical Analysis
- 2. Write a reasonable mechanism that converts the reactants into the products. Avoid issues A-U from the previous page. You can use any number of steps (it does not have to be a one-step mechanism). Do not use any other chemicals (solvents, acids, bases, etc.) in your mechanism. 2 2 H ΗΘarrow_forwardFor the following reaction, the partial pressures were determined for the reaction components as shownbelow. Is the reaction at equilibrium? If not, in which direction will it proceed?I2 (g) + Cl2 (g) ⇋ 2 ICl (g) Kp = 81.9 partial pressures: I2 = 0.114 atm; Cl2 = 0.102 atm; ICl = 0.355 atmarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. H3O+ + Oarrow_forward
- Write the law of mass action for the following quilibrium 2N2O5(g) --> 4 NO2(g) + O2(g).arrow_forwardState the products (formulas) of the reaction of acetophenone with iodine and NaOH.arrow_forwardCh. 4- Precipitation Reactions Worksheet Write balanced, complete ionic, and net ionic equations for the following reactions that mav produce precipitates. Use NR to indicate no reaction Ave 1\ +3 =6 Fe + V-2 Na S04 13. Write the balanced equation for the reaction of iron (III) phosphate with sodium sulfate to make iron (III) sulfate and sodium phosphate. 2FePO4 + M, Soy a) If you perform this reaction with 25 grams of iron (III) phosphate and an excess of sodium sulfate, how many grams of iron (III) sulfate can you make? 21 Fe 2 3x 1 Na 3 25g Fe Ingle 150,829 Indes 2 nol 3 1335 349.89 35.90 Ihol & Sanz Fez Bak heck 3x1 50ab) If 18.5 grams of iron (III) sulfate are actually made when you do this reaction, what is your Poy percent yield? 118.5 259-1-100 51.4% (0.74)x100610 335 If you do this reaction with 15 grams of sodium sulfate and get a 65.0% yield, how many grams of sodium phosphate will you make? 10.59 14. Ammonia is produced from the reaction of nitrogen and hydrogen according…arrow_forward
- == Functional Groups Identifying and drawing hemiacetals and acetals In the drawing area below, create an acetal with 1 isopropoxy group, 1 hydroxyl group, and a total of 10 carbon atoms. Explanation Click and drag to start drawing a structure. Check G +arrow_forwardState the products (formulas) of the reaction of acetophenone with iodine and NaOH.arrow_forwardExplanation Check Draw the skeletal ("line") structure of 5-hydroxy-4-methyl-2-pentanone. Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cer ☐ : Carrow_forward
- 1. Using a Model set Build a model for the following compound [CH2BrCI]. 2. Build another model of the mirror image of your first molecule. 3. Place the two models next to each other and take a picture which shows the differences between the two models. 4. Determine the absolute stereochemistry R or S for the two models. 5. Write or type a paragraph to Discuss the stereochemical relationship between the two models of CH2BrCl. You must provide an explanation for your conclusions also provide a description for the colors used to represent each atom in the model's images.arrow_forwardWhat parameters are included in the specific rotation calculation of a pure substance based on measurement from a polarimeter? Select one or more: Density of the sample Pathlength of the sample container Enantiomeric excess of the sample Measured rotation of lightarrow_forwardV Determine whether the following molecule is a hemiacetal, acetal, or neither and select the appropriate box below. Also, highlight the hemiacetal or acetal carbon if there is one. Explanation O CH O Ohemiacetal Oacetal Oneither Check A 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Cer 000 Ararrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





