
Organic Chemistry
8th Edition
ISBN: 9781337516402
Author: Brown
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.15P
Following are three compounds with the molecular formula C4H8O2 and three 1H-NMR spectra. Assign each compound its correct spectrum and assign all signals to their corresponding hydrogens.
(1) 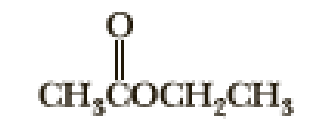
(2) 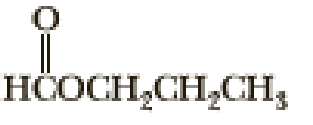
(3) 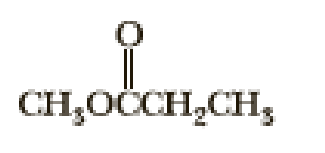
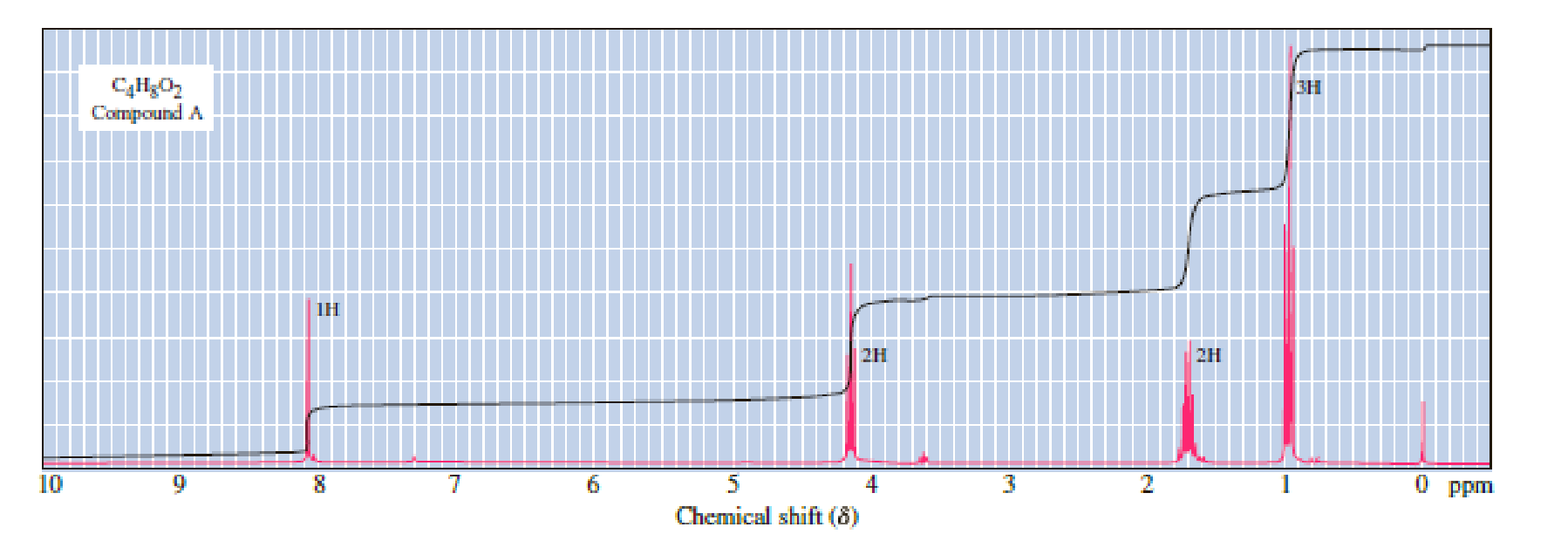
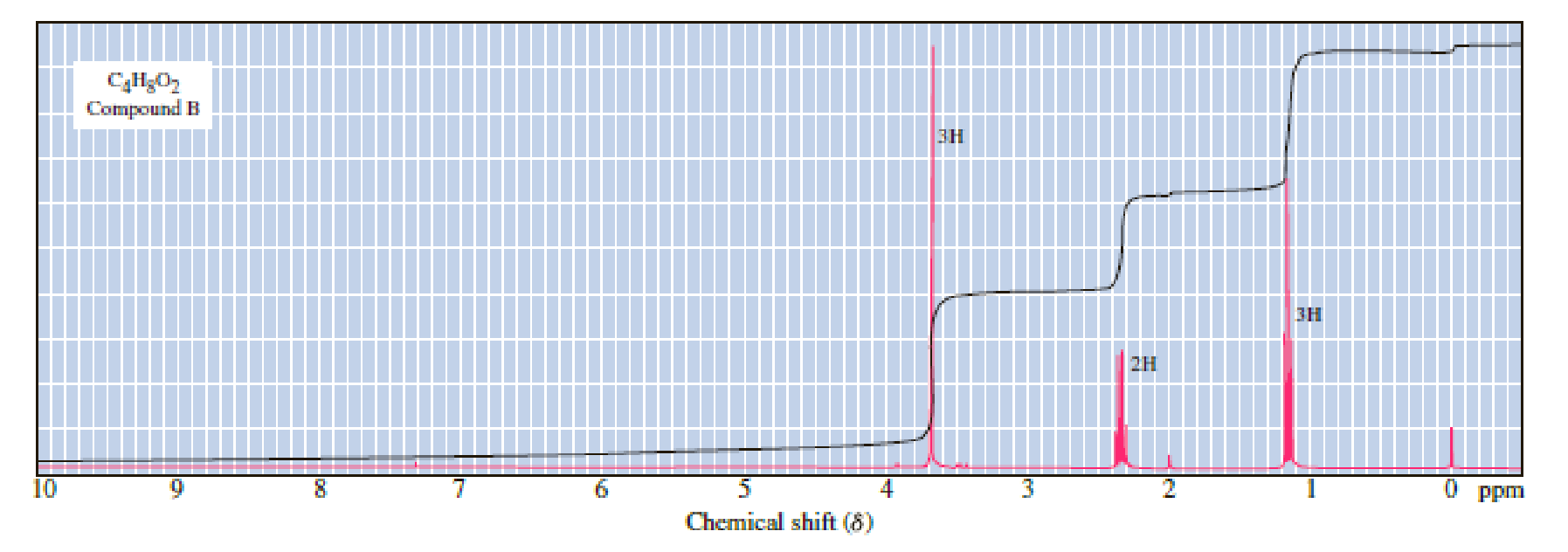
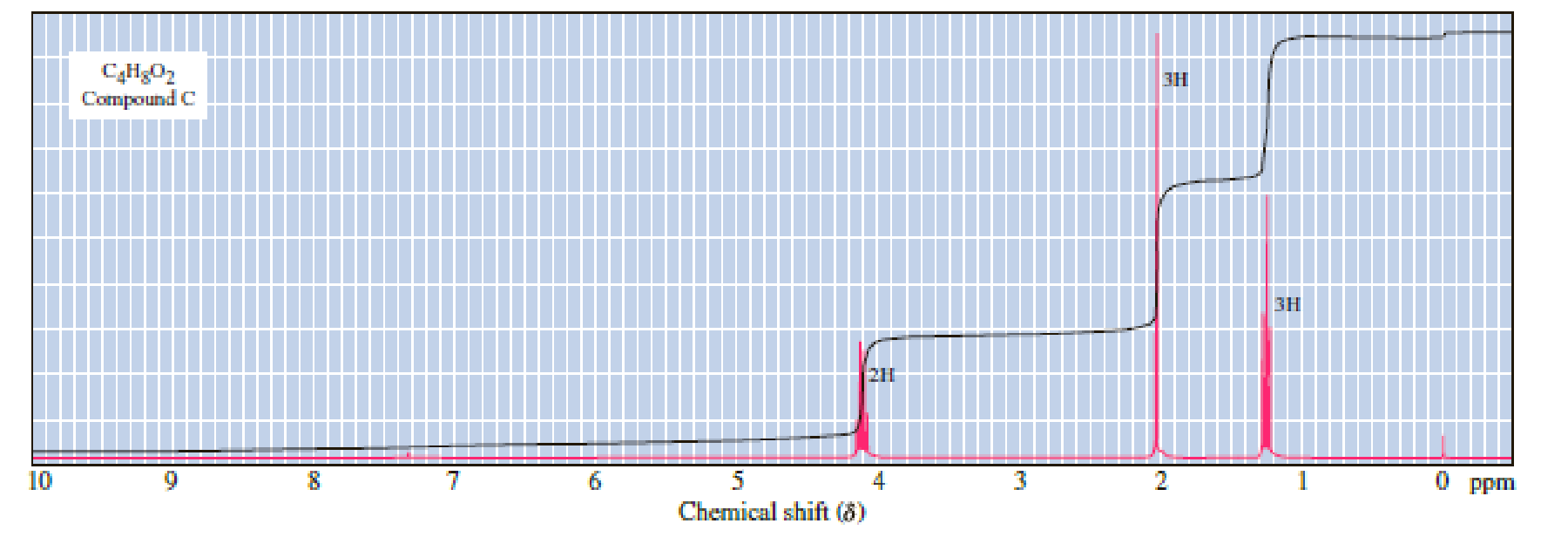
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
how many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20
calculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2
an adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank provide
Chapter 13 Solutions
Organic Chemistry
Ch. 13.2 - Calculate the ratio of nuclei in the higher spin...Ch. 13.5 - State the number of sets of equivalent hydrogens...Ch. 13.5 - Each compound gives only one signal in its 1H-NMR...Ch. 13.6 - The line of integration of the two signals in the...Ch. 13.7 - Following are two constitutional isomers with the...Ch. 13.8 - Following are pairs of constitutional isomers....Ch. 13.10 - Following is a 1H-NMR spectrum of 2-butanol....Ch. 13.11 - Explain how to distinguish between the members of...Ch. 13 - Prob. 13.9PCh. 13 - Prob. 13.10P
Ch. 13 - Prob. 13.11PCh. 13 - Following are structural formulas for three...Ch. 13 - Following arc structural formulas for the cis...Ch. 13 - Prob. 13.14PCh. 13 - Following are three compounds with the molecular...Ch. 13 - Following are 1H-NMR spectra for compounds D, E,...Ch. 13 - Following are 1H-NMR spectra for compounds G, H,...Ch. 13 - Propose a structural formula for compound J,...Ch. 13 - Compound K, molecular formula C6H14O, readily...Ch. 13 - Compound M, molecular formula C5H10O, readily...Ch. 13 - Following is the 1H-NMR spectrum of compound O,...Ch. 13 - Treatment of compound P with BH3 followed by...Ch. 13 - The 1H-NMR spectrum of compound R, C6H14O,...Ch. 13 - Write structural formulas for the following...Ch. 13 - Prob. 13.25PCh. 13 - Ascaridole is a natural product that has been used...Ch. 13 - The 13C-NMR spectrum of 3-methyl-2-butanol shows...Ch. 13 - Prob. 13.28P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO2 (g) = N2O4(g) AGº = -5.4 kJ Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system: Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to '2' rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. 00 rise ☐ x10 fall yes no ☐ atm G Ar 1arrow_forwardWhy do we analyse salt?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H H CH3OH, H+ H Select to Add Arrows H° 0:0 'H + Q HH ■ Select to Add Arrows CH3OH, H* H. H CH3OH, H+ HH ■ Select to Add Arrows i Please select a drawing or reagent from the question areaarrow_forward
- What are examples of analytical methods that can be used to analyse salt in tomato sauce?arrow_forwardA common alkene starting material is shown below. Predict the major product for each reaction. Use a dash or wedge bond to indicate the relative stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts H Šali OH H OH Select to Edit Select to Draw 1. BH3-THF 1. Hg(OAc)2, H2O =U= 2. H2O2, NaOH 2. NaBH4, NaOH + Please select a drawing or reagent from the question areaarrow_forwardWhat is the MOHR titration & AOAC method? What is it and how does it work? How can it be used to quantify salt in a sample?arrow_forward
- Predict the major products of this reaction. Cl₂ hv ? Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. Note for advanced students: you can ignore any products of repeated addition. Explanation Check Click and drag to start drawing a structure. 80 10 m 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility DII A F1 F2 F3 F4 F5 F6 F7 F8 EO F11arrow_forwardGiven a system with an anodic overpotential, the variation of η as a function of current density- at low fields is linear.- at higher fields, it follows Tafel's law.Calculate the range of current densities for which the overpotential has the same value when calculated for both cases (the maximum relative difference will be 5%, compared to the behavior for higher fields).arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AGº = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of N will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Х ด ? olo 18 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY