
Concept explainers
a)
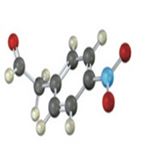
Interpretation:
IR absorptions for the molecules are interpreted and stated.
Concept introduction:
IR absorption is used to identify the
Answer to Problem 12VC
The molecules IR absorptions is 3100,1730,1450,1600 and 1540 cm-1.
Explanation of Solution
a) In the molecule C-H stretch shows an absorption at 3100 cm-1 , C=C
The molecules IR absorptions is 3100,1730,1450,1600 and 1540 cm-1.
b)
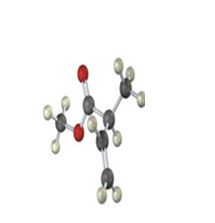
Interpretation:
IR absorptions for the molecules are interpreted and stated.
Concept introduction:
IR absorption is used to identify the functional groups of the molecule.
Answer to Problem 12VC
The molecules IR absorptions is 2850-2960,1715,920 cm-1.
Explanation of Solution
In the molecule
The molecules IR absorptions is 2850-2960,1715,920 cm-1.
c)
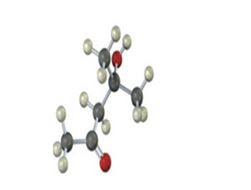
Interpretation:
IR absorptions for the molecules are interpreted and stated.
Concept introduction:
IR absorption is used to identify the functional groups of the molecule.
Answer to Problem 12VC
The molecules IR absorptions is 2850-2960,1715,3500 cm-1.
Explanation of Solution
In the molecule alkane C-H stretch shows an absorption at 2850-2960 cm-1 , O-H stretching at 3500 cm-1. The carbonyl C=O shows an absorption at 1715 cm-1.
The molecules IR absorptions is 2850-2960,1715,3500 cm-1.
Want to see more full solutions like this?
Chapter 12 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- Write the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forwardtheres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forward
- Draw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forwardOrganic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forward
- An aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forwardDraw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forwardAnswer the question in the first photoarrow_forward
- Ggggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forwardBriefly describe a nucleophilic addition.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


