
Concept explainers
a)
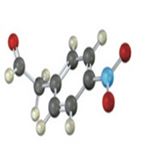
Interpretation:
IR absorptions for the molecules are interpreted and stated.
Concept introduction:
IR absorption is used to identify the
Answer to Problem 12VC
The molecules IR absorptions is 3100,1730,1450,1600 and 1540 cm-1.
Explanation of Solution
a) In the molecule C-H stretch shows an absorption at 3100 cm-1 , C=C
The molecules IR absorptions is 3100,1730,1450,1600 and 1540 cm-1.
b)
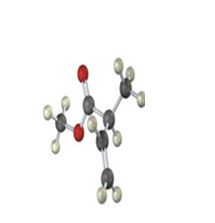
Interpretation:
IR absorptions for the molecules are interpreted and stated.
Concept introduction:
IR absorption is used to identify the functional groups of the molecule.
Answer to Problem 12VC
The molecules IR absorptions is 2850-2960,1715,920 cm-1.
Explanation of Solution
In the molecule
The molecules IR absorptions is 2850-2960,1715,920 cm-1.
c)
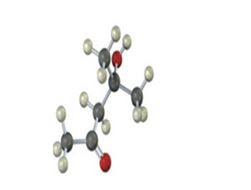
Interpretation:
IR absorptions for the molecules are interpreted and stated.
Concept introduction:
IR absorption is used to identify the functional groups of the molecule.
Answer to Problem 12VC
The molecules IR absorptions is 2850-2960,1715,3500 cm-1.
Explanation of Solution
In the molecule alkane C-H stretch shows an absorption at 2850-2960 cm-1 , O-H stretching at 3500 cm-1. The carbonyl C=O shows an absorption at 1715 cm-1.
The molecules IR absorptions is 2850-2960,1715,3500 cm-1.
Want to see more full solutions like this?
Chapter 12 Solutions
EP ORGANIC CHEMISTRY,24 MONTH-OWLV2
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


