
ORGANIC CHEMISTRY W/BIOLOGICAL TOPICS
6th Edition
ISBN: 9781260325294
Author: SMITH
Publisher: RENT MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12C.9, Problem 23P
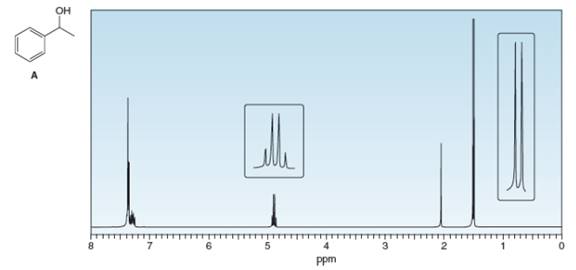
What protons in alcohol A give rise to each signal in its
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff:
a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight
of
pina colone
Mechanism
For
b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture
(3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable
the formation of the products
what are the Iupac names for each structure
What are the IUPAC Names of all the compounds in the picture?
Chapter 12C Solutions
ORGANIC CHEMISTRY W/BIOLOGICAL TOPICS
Ch. 12C.1 - Problem 14.1 The NMR spectrum of recorded on a ...Ch. 12C.1 - Prob. 2PCh. 12C.2 - How many 1H NMR signals does each...Ch. 12C.2 - How many 1H NMR signals does each compound give?...Ch. 12C.2 - Label the protons in each highlighted CH2 group as...Ch. 12C.2 - How many 1H NMR signals would you expect for each...Ch. 12C.3 - Prob. 9PCh. 12C.3 - For each compound, first label each different type...Ch. 12C.3 - Label each statement as True or False. a. When a...Ch. 12C.4 - Prob. 12P
Ch. 12C.5 - Problem 14.12 Which compound give a NMR spectrum...Ch. 12C.5 - Prob. 14PCh. 12C.6 - Prob. 15PCh. 12C.6 - For each compound give the number of 1H NMR...Ch. 12C.6 - Prob. 17PCh. 12C.7 - Prob. 18PCh. 12C.7 - Problem 14.18 Describe the NMR spectrum of each...Ch. 12C.8 - Problem 14.19 Draw a splitting diagram for in ,...Ch. 12C.8 - Problem 14.20 Identify A and B, isomers of...Ch. 12C.9 - Problem 14.21 How many signals are present in the ...Ch. 12C.9 - Problem 14.22 What protons in alcohol A give rise...Ch. 12C.9 - How many peaks are observed in the NMR signal for...Ch. 12C.10 -
Problem 14.24 Propose a structure for a compound...Ch. 12C - 14.34 (a) How many NMR signals does each of the...Ch. 12C - 14.35 (a) How many NMR signals does each compound...Ch. 12C - Prob. 38PCh. 12C - 14.37 How many NMR signals does each natural...Ch. 12C - Prob. 40PCh. 12C - 14.39 What effect does increasing the operating...Ch. 12C - Prob. 43PCh. 12C - Prob. 44PCh. 12C - Prob. 45PCh. 12C - Prob. 47PCh. 12C - Prob. 48PCh. 12C - 14.48 How many NMR signals does each compound...Ch. 12C - 14.49 Rank the highlighted carbon atoms in each...Ch. 12C - 14.50 Identify the carbon atoms that give rise to...Ch. 12C - 14.62 Reaction of with , followed by treatment...Ch. 12C - Reaction of aldehyde D with amino alcohol E in the...Ch. 12C - 14.64 Propose a structure consistent with each set...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Give the IUPAC name for each compound.
Organic Chemistry
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
What are the cervical and lumbar enlargements?
Principles of Anatomy and Physiology
1. Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
the interactio...
Biochemistry: Concepts and Connections (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1) a) Give the dominant Intermolecular Force (IMF) in a sample of each of the following compounds. Please show your work. (8) SF2, CH,OH, C₂H₂ b) Based on your answers given above, list the compounds in order of their Boiling Point from low to high. (8)arrow_forward19.78 Write the products of the following sequences of reactions. Refer to your reaction road- maps to see how the combined reactions allow you to "navigate" between the different functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18 roadmaps along with your new Chapter 19 roadmap for these. (a) 1. BHS 2. H₂O₂ 3. H₂CrO4 4. SOCI₂ (b) 1. Cl₂/hv 2. KOLBU 3. H₂O, catalytic H₂SO4 4. H₂CrO4 Reaction Roadmap An alkene 5. EtOH 6.0.5 Equiv. NaOEt/EtOH 7. Mild H₂O An alkane 1.0 2. (CH3)₂S 3. H₂CrO (d) (c) 4. Excess EtOH, catalytic H₂SO OH 4. Mild H₂O* 5.0.5 Equiv. NaOEt/EtOH An alkene 6. Mild H₂O* A carboxylic acid 7. Mild H₂O* 1. SOC₁₂ 2. EtOH 3.0.5 Equiv. NaOEt/E:OH 5.1.0 Equiv. NaOEt 6. NH₂ (e) 1. 0.5 Equiv. NaOEt/EtOH 2. Mild H₂O* Br (f) i H An aldehyde 1. Catalytic NaOE/EtOH 2. H₂O*, heat 3. (CH,CH₂)₂Culi 4. Mild H₂O* 5.1.0 Equiv. LDA Br An ester 4. NaOH, H₂O 5. Mild H₂O* 6. Heat 7. MgBr 8. Mild H₂O* 7. Mild H₂O+arrow_forwardLi+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water? Group of answer choices LiBr LiI LiF LiClarrow_forward
- Q4: Write organic product(s) of the following reactions and show the curved-arrow mechanism of the reactions. Br MeOH OSO2CH3 MeOHarrow_forwardProvide the correct IUPAC name for the compound shown here. Reset cis- 5- trans- ☑ 4-6- 2- 1- 3- di iso tert- tri cyclo sec- oct but hept prop hex pent yl yne ene anearrow_forwardQ6: Predict the major product(s) for the following reactions. Note the mechanism (SN1, SN2, E1 or E2) the reaction proceeds through. If no reaction takes place, indicate why. Pay attention to stereochemistry. NaCN DMF Br σ Ilm... Br H Br H H NaCN CH3OH KOtBu tBuOH NaBr H₂O LDA Et2O (CH3)2CHOH KCN DMSO NaOH H₂O, A LDA LDA Systemarrow_forward
- Q7: For the following reactions, indicate the reaction conditions that would provide the indicated product in a high yield. Note the major reaction pathway that would take place (SN1, SN2, E1, or E2) Note: There may be other products that are not shown. There maybe more than one plausible pathway. Br H3C OH H3C CI ... H3C SCH2CH3 CI i SCH2CH3 ཨ་ Br System Settarrow_forwardQ2: Rank the compounds in each of the following groups in order of decreasing rate of solvolysis in aqueous acetone. OSO2CF3 OSO2CH3 OH a. b. CI Brarrow_forwardох 4-tert-butyl oxy cyclohex-1-ene Incorrect, 1 attempt remaining The systematic name of this compound classifies the -OR group as a substituent of the hydrocarbon, which is considered the principal functional group. The ether substituent is named with the suffix 'oxy'. The general format for the systematic name of a hydrocarbon is: [prefix/substituent] + [parent] + [functional group suffix] Substituents are listed in alphabetical order. Molecules with a chiral center will indicate the absolute configuration at the beginning of its name with the R and S notation.arrow_forward
- 5. Compressibility (6 points total). The isothermal compressibility is a measure of how hard/easy it is to compress an object (how squishy is it?) at constant temperature. It is др defined as Br=-()=-(200²)T' (a) You might wonder why there is a negative sign in this formula. What does it mean when this quantity is positive and what does it mean when this quantity is negative? (b) Derive the formula for the isothermal compressibility of an ideal gas (it is very simple!) (c) Explain under what conditions for the ideal gas the compressibility is higher or lower, and why that makes sense.arrow_forward19. (3 pts) in Chapter 7 we will see a reaction of halocyclohexanes that requires that the halogen occupy an axial position with this in mind, would you expect cis-1-bromo-3-methylcyclohexane or trans-1-bromo-3-methylcyclohexane to be more reactive in this reaction? Briefly explain your choice using structures to support your answer. Mere-eries-cecleone) The tran-i-browse-3-methylcyclohexionearrow_forwardPlease help me calculate the undiluted samples ppm concentration. My calculations were 280.11 ppm. Please see if I did my math correctly using the following standard curve. Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EVSJL_W0qrxMkUjK2J3xMUEBHDu0UM1vPKQ-bc9HTcYXDQ?e=hVuPC4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY