
CNCT ORG CHEM 6 2020
6th Edition
ISBN: 9781266807244
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12A.2, Problem 7P
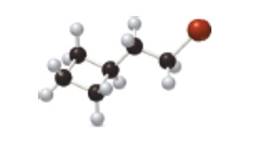
What molecular ions would you expect for the compound depicted in the ball-and-stick model?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which will evaporate faster, 1-Butanol or Pentane? Explain your choice.
Using the equation below, what is the rate of this reaction if the rate of disappearance of H2 is 0.44 M/sec?
H2 + Br2 → 2HBr
2Fe3+(aq) + Sn2+(aq) □ 2Fe²+(aq) + Sn 4+ (aq)
If the change in Sn²+ concentration is 0.0010M in 38.5 seconds, what is the rate of disappearance of
Sn²+?
Chapter 12A Solutions
CNCT ORG CHEM 6 2020
Ch. 12A.1 - What is the mass of the molecular ion formed from...Ch. 12A.1 - Prob. 3PCh. 12A.2 - Prob. 6PCh. 12A.2 - What molecular ions would you expect for the...Ch. 12A.3 - The mass spectrum of 2,3-dimethylpentane also...Ch. 12A.3 - The base peak in the mass spectrum of 2, 2,...Ch. 12A - Problem-13.24 The mass spectrum of the following...Ch. 12A - Prob. 16PCh. 12A - Which compound gives a molecular ion at m/z= 122,...Ch. 12A - Propose two molecular formulas for each molecular...
Ch. 12A - Propose four possible structures for a hydrocarbon...Ch. 12A - Problem-13.29 What is the molecular formula for...Ch. 12A - Problem-13.30 Propose a molecular formula for rose...Ch. 12A - 13.31 Match each structure to its mass spectrum
Ch. 12A - 13.32 Propose two possible structures for a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For a neutral hydrogen atom with an electron in the n = 4 state, how many different energies are possible when a photon is emitted? 4 3 2 1 There are infinite possibilitiesarrow_forward2 NO(g) + H2(g) → N2(g) +2 H2O(g) If NO has rate of disappearance of 0.025 M/min, what is the rate of this reaction?arrow_forward2Fe3+(aq) + Sn2+(aq) □ 2Fe²+(aq) + Sn 4+ (aq) If the change in Sn2+ concentration is 0.0010M in 38.5 seconds, what is the rate of appearance of Fe²+?arrow_forward
- Using the equation below, if the rate of disappearance of Cl2 is 0.26 M/min, what is the rate of this reaction? 2NO(g) + Cl2(g) → 2NOCI(g)arrow_forwardA 45.0 mL solution containing a mixture of 0.0634 M KCN and 0.0634 M KCI is titrated with 0.107 M AgNO. From this mixture, which silver salt will precipitate first? A list of Ksp values can be found in the table of solubility constants. • AgCI • not enough information to determine AgCN What is the concentration of Ag* at the first equivalence point? [Ag*] = Will the second silver salt begin to precipitate at the first equivalence point before the first silver salt has completely precipitated? • not enough information to determine • yes • noarrow_forward[Review Topics] [References] Indicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different conformations of the same compound, or the same conformation of a compound viewed from a different perspective. Note that cis, trans isomers are an example of stereoisomers. H₂N ✓ CI H₂N NH2 NH₂ CI Submit Answer Retry Entire Group 2 more group attempts remaining Previous Next>arrow_forward
- Don't used Ai solutionarrow_forwardDraw resonance structures for the following compounds. Please provide a thorough explanation that allows for undertanding of topic.arrow_forwardBF3 has a no dipole moment. a) Draw the Lewis structure for BF3, showing all nonbonding electrons. b) Indicate the polarity of every atom in the structure using δ+ and δ– notation, and explain why the molecule has no net dipole. Please provide a thorough explanation that allows for undertanding of topic.arrow_forward
- For each reaction shown below follow the curved arrows to complete each equation by showing the structure of the products. Identify the acid, the base, the conjugated acid and conjugated base. Consutl a pKa table and choose the direciton the equilibrium goes. Please provide a thorough explanation that allows for undertanding of topic.arrow_forwardNeed help understanding please help Let’s assume the initial volume of the gas is 4.80 LL , the initial temperature of the gas is 29.0 °C°C , and the system is in equilibrium with an external pressure of 1.2 bar (given by the sum of a 1 bar atmospheric pressure and a 0.2 bar pressure due to a brick that rests on top of the piston). What is the final pressure of the gas? What is the final volume of the gas? What happens with the piston after you finish heating the gas? Assume you do not need to worry about the gas cooling down again because the outside of the container is at a lower temperature. That is, you manage to keep the gas at a constant temperature that equals 54.2 °C°C What is the sign of w? What is the value of w? Be careful with units. How do you convert bar*L to J?arrow_forwardFor a neutral hydrogen atom with an electron in the n = 4 state, how many different energies are possible when a photon is emitted?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks ColeChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks ColeChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Periodic Properties of Elements | Chemistry | IIT-JEE | NEET | CBSE | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=L26rRWz4_AI;License: Standard YouTube License, CC-BY
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE; Author: Melissa Maribel;https://www.youtube.com/watch?v=0h8q1GIQ-H4;License: Standard YouTube License, CC-BY