
Concept explainers
(a)
Interpretation:
Mechanism for the tautomerization process that occurs under acidic conditions has to be drawn.
Concept Introduction:
Keto-enol tautomerization occurs when a hydroxyl group is present next to a double bond in presence of acid or base. Due to this the

Acid catalyzed tautomerization:
Compounds that are ketone can undergo keto-enol tautomerization in presence of acid. Tautomers are not same as resonance structures. The chemical properties of tautomers will be different. A general mechanism of keto-enol tautomerization that occur in acidic condition can be given as,
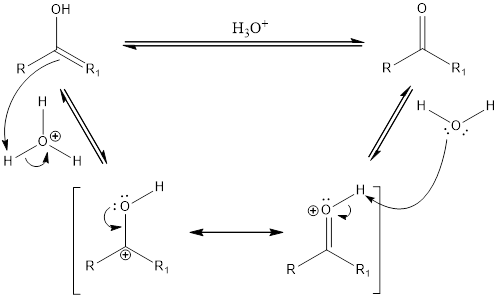
First step is the formation of carbocation. Second step is the formation of double bond between oxygen and carbon atom. The water acts as a base and abstracts a proton to form the final keto compound.
(b)
Interpretation:
Mechanism for the tautomerization process that occurs under basic conditions has to be drawn.
Concept Introduction:
Keto-enol tautomerization occurs when a hydroxyl group is present next to a double bond in presence of acid or base. Due to this the ketone will have few amount of enol also. The quantity of ketone and enol is determined only by equilibrium.

Base catalyzed tautomerization:
Compounds that are ketone can undergo keto-enol tautomerization in presence of acid. Tautomers are not same as resonance structures. The chemical properties of tautomers will be different. A general mechanism of keto-enol tautomerization that occur in basic condition can be given as,
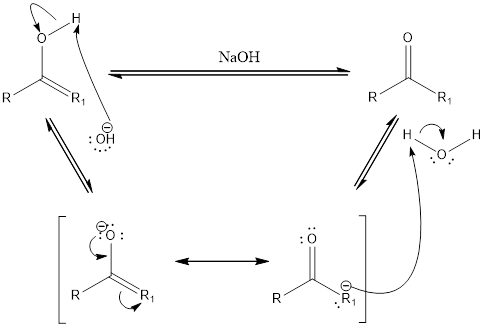
First step is the removal of proton. Second step is the formation of double bond between oxygen and carbon atom. The water acts as an acid and donates a proton to form the final keto compound.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry As a Second Language: First Semester Topics
- Draw everything please on a piece of paper explaining each steparrow_forwardDefine crystalline, polycrystalline and amorphous materials What crystal system and Bravais lattices are shown in the figure immediately below? What do a, b, C, a, ẞ and y represent and what are their values? You can label the Bravais lattices directly above or under the figure. C aarrow_forward32. The diagrams below show the band structure of an intrinsic semiconductor at absolute zero and room temperature. Room Temperature EF E OK Ep- a) In the space below, sketch a similar pair of diagrams for an n-type semiconductor. D) Give the definition and an example of (i) an intrinsic semiconductor and (ii) an n-type semiconductor.arrow_forward
- 29. a) i Which energy diagram best represents the d-electrons in tetrahedral [Co(NH3)4]²+? b) ii c) iii d) iv 11 ་ ↑↓ ↑t t ↑↓ ↑↓ e) none of these ii In1 According to Slater's rules, what is the effective nuclear charge experienced by a 3d electron in 30. Ge? a) 32.00 b) 21.15 c) 16.05 d) 14.00 e) 10.85arrow_forwardRegarding Lowis structuros and geometrios, Draw Lewis structures for the following: SOF4, SO, ICI, XeO2F4, SeF and XeO3. For each one, indicate the observed molecular geometry it adopts.arrow_forwardExplain the following statements with equations: - The fusion product of an organic compund with sodium metal is an alkaline solution - The test for elements should be done before the solubility tests. - Using less sodium than the organic compound in the ignition test might cause a problem to detect the presence of the nitrogen and sulfur - Formation of colored product when adding ferric acid chloride to phenol solutionarrow_forward
- 31 Indicate the symbol, mass number and the atomic number of the missing product in each of the following nuclear reactions. a) 13/3 N 41 b) 11 Ca 20 c) 90 38 Sr → 133 C + ? + - 6 0 e →? 90 Y + ? 39 11 d) 22 Na → ? + + 1 B +1 β Toarrow_forwardPlease drawarrow_forward9. compore the Following two Venctions IN termy Of Ronction Rate and explan in detail the reasoning that led to your conclusion +He p₁₂ 11- ㅐ 15 .. +He H #H H / H b. Compare the Following too reactions 14 terms of reaction Rate and explain in detail the reasoning that led to your conclusion Н d-C- tłu Na +2446 е -ll +2n "Harrow_forward
- a. •Write all of the possible products For the Following ronction А ----- H - H H + H₂0 H+ Н b. in Rite the complete reaction Mechaniszn For the Formation of each product. ·C. Suggest what Reaction conditions could Result in each product being the major Product of the veaction:arrow_forwarda. Write the product For each of the Following reactions H 6-836-6 레 +H₂ N A H A-C-C=C-C-CH + 2 Na +2 NH3 - H H b. Write the reaction Mechanism For. reaction eacharrow_forwardhelp draw the moleculearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





