
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
7th Edition
ISBN: 9780134240152
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.3, Problem 7P
Muscalure is the sex attractant of the common housefly. Flies are lured to traps filled with bait that contain muscalure and an insecticide. Eating the bait is fatal. How could you synthesize muscalure using 1-bromopentane as one of the starting materials?
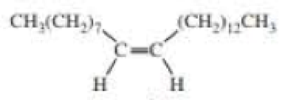
muscalure
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.
The quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.
The quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.
Chapter 12 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
Ch. 12.1 - Prob. 1PCh. 12.2 - Which is more reactive an organolithium compound...Ch. 12.2 - Prob. 3PCh. 12.3 - PROBLEM 6♦
Explain why tertiary alkyl halides...Ch. 12.3 - Muscalure is the sex attractant of the common...Ch. 12.3 - Prob. 8PCh. 12.3 - Prob. 9PCh. 12.3 - Prob. 10PCh. 12.4 - Prob. 13PCh. 12.4 - Prob. 14P
Ch. 12.4 - Prob. 15PCh. 12.4 - Show how the Suzuki and/or Heck reactions can be...Ch. 12.4 - Identify two pairs of an alkyl bromide and an...Ch. 12.5 - Prob. 19PCh. 12.5 - Draw the product of ring-closing metathesis for...Ch. 12.5 - Prob. 22PCh. 12 - Prob. 23PCh. 12 - Prob. 24PCh. 12 - Identify A through H.Ch. 12 - 26. Using the given starting material, any...Ch. 12 - Prob. 27PCh. 12 - Prob. 28PCh. 12 - Prob. 29PCh. 12 - Using ethynyleyclohexane as a starting material...Ch. 12 - Prob. 31PCh. 12 - Using the given starting material, any necessary...Ch. 12 - Prob. 33PCh. 12 - A student added an equivalent of...Ch. 12 - Using the given starting material, any necessary...Ch. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - Bombykol is the sex pheromone of the silk moth....Ch. 12 - Prob. 39PCh. 12 - A dibromide loses only one bromine when it reacts...Ch. 12 - What starting material is required in order to...Ch. 12 - Prob. 42PCh. 12 - Prob. 1PCh. 12 - Prob. 2PCh. 12 - Prob. 3PCh. 12 - Prob. 4P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
- For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forwardWrite the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.arrow_forwardName the following using IUPAC.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License