
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
thumb_up100%
Chapter 12.3, Problem 12.2QQ
Identify the paths A, B, C, and D in Figure 12.11 as isobaric, isothermal, isovolumetric, or adiabatic. For path B, Q = 0.
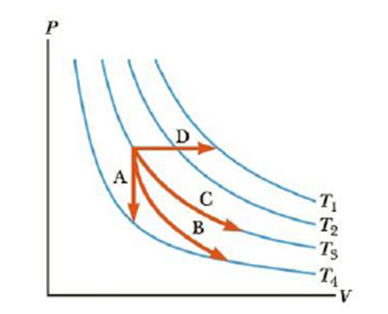
Figure 12.11 (Quick Quiz 12.2) Identify the nature of paths A, B, C, and D
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Hi,
I have canceled, why did you charge me again?
No chatgpt pls will upvote
No chatgpt pls will upvote
Chapter 12 Solutions
College Physics
Ch. 12.1 - By visual inspection, order the PV diagrams shown...Ch. 12.3 - Identify the paths A, B, C, and D in Figure 12.11...Ch. 12.4 - Three engines operate between reservoirs separated...Ch. 12.5 - Which of the following is true for the entropy...Ch. 12.5 - Prob. 12.5QQCh. 12 - Two identical containers each hold 1 mole of an...Ch. 12 - Which one of the following statements is true? (a)...Ch. 12 - Prob. 3CQCh. 12 - Prob. 4CQCh. 12 - For an ideal gas in an isothermal process, there...
Ch. 12 - An ideal gas undergoes an adiabatic process so...Ch. 12 - Is it possible to construct a heat engine that...Ch. 12 - A heat engine does work Weng while absorbing...Ch. 12 - When a sealed Thermos bottle full of hot coffee is...Ch. 12 - The first law of thermodynamics is U = Q + W. For...Ch. 12 - The first law of thermodynamics says we cant get...Ch. 12 - Objects A and B with TA TB are placed in thermal...Ch. 12 - Prob. 13CQCh. 12 - Prob. 14CQCh. 12 - An ideal gas is compressed to half its initial...Ch. 12 - A thermodynamic process occurs in which the...Ch. 12 - Prob. 17CQCh. 12 - An ideal gas is enclosed in a cylinder with a...Ch. 12 - Sketch a PV diagram and find the work done by the...Ch. 12 - Gas in a container is at a pressure of 1.5 atm and...Ch. 12 - Find the numeric value of the work done on the gas...Ch. 12 - A gas expands from I to F along the three paths...Ch. 12 - A gas follows the PV diagram in Figure P12.6. Find...Ch. 12 - A sample of helium behaves as an ideal gas as it...Ch. 12 - (a) Find the work done by an ideal gas as it...Ch. 12 - One mole of an ideal gas initially at a...Ch. 12 - (a) Determine the work done on a fluid that...Ch. 12 - A balloon holding 5.00 moles of helium gas absorbs...Ch. 12 - A chemical reaction transfers 1250 J of thermal...Ch. 12 - Prob. 13PCh. 12 - A cylinder of volume 0.300 m3 contains 10.0 mol of...Ch. 12 - A gas expands from I to F in Figure P12.5. The...Ch. 12 - In a running event, a sprinter does 4.8 105 J of...Ch. 12 - A gas is compressed at a constant pressure of...Ch. 12 - A quantity of a monatomic ideal gas undergoes a...Ch. 12 - A gas is enclosed in a container fitted with a...Ch. 12 - A monatomic ideal gas under-goes the thermodynamic...Ch. 12 - An ideal gas is compressed from a volume of Vi =...Ch. 12 - A system consisting of 0.025 6 moles of a diatomic...Ch. 12 - An ideal monatomic gas expands isothermally from...Ch. 12 - An ideal gas expands at constant pressure. (a)...Ch. 12 - An ideal monatomic gas contracts in an isobaric...Ch. 12 - An ideal diatomic gas expands adiabatically from...Ch. 12 - An ideal monatomic gas is contained in a vessel of...Ch. 12 - Consider the cyclic process described by Figure...Ch. 12 - A 5.0-kg block of aluminum is heated from 20C to...Ch. 12 - One mole of gas initially at a pressure of 2.00...Ch. 12 - A gas increases in pressure from 2.00 atm to 6.00...Ch. 12 - An ideal gas expands at a constant pressure of...Ch. 12 - A heat engine operates between a reservoir at 25C...Ch. 12 - A heat engine is being designed to have a Carnot...Ch. 12 - The work done by an engine equals one-fourth the...Ch. 12 - In each cycle of its operation, a heat engine...Ch. 12 - One of the most efficient engines ever built is a...Ch. 12 - A lawnmower engine ejects 1.00 104 J each second...Ch. 12 - An engine absorbs 1.70 kJ from a hot reservoir at...Ch. 12 - A heat pump has a coefficient of performance of...Ch. 12 - A freezer has a coefficient of performance of...Ch. 12 - Prob. 42PCh. 12 - In one cycle a heat engine absorbs 500 J from a...Ch. 12 - A power plant has been proposed that would make...Ch. 12 - Prob. 45PCh. 12 - A heat engine operates in a Carnot cycle between...Ch. 12 - A Styrofoam cup holding 125 g of hot water at 1.00...Ch. 12 - A 65-g ice cube is initially at 0.0C. (a) Find the...Ch. 12 - A freezer is used to freeze 1.0 L of water...Ch. 12 - What is the change in entropy of 1.00 kg of liquid...Ch. 12 - A 70.0-kg log falls from a height of 25.0 m into a...Ch. 12 - A sealed container holding 0.500 kg of liquid...Ch. 12 - Prob. 53PCh. 12 - When an aluminum bar is temporarily connected...Ch. 12 - Prepare a table like Table 12.3 for the following...Ch. 12 - Prob. 56PCh. 12 - Prob. 57PCh. 12 - Prob. 58PCh. 12 - Sweating is one of the main mechanisms with which...Ch. 12 - Prob. 60PCh. 12 - Suppose a highly trained athlete consumes oxygen...Ch. 12 - A Carnot engine operates between the temperatures...Ch. 12 - Prob. 63APCh. 12 - A Carnot engine operates between 100C and 20C. How...Ch. 12 - A substance undergoes the cyclic process shown in...Ch. 12 - When a gas follows path 123 on the PV diagram in...Ch. 12 - Prob. 67APCh. 12 - An ideal gas initially at pressure P0, volume V0,...Ch. 12 - One mole of neon gas is heated from 300. K to 420....Ch. 12 - Every second at Niagara Falls, approximately 5.00 ...Ch. 12 - A cylinder containing 10.0 moles of a monatomic...Ch. 12 - Prob. 72APCh. 12 - Suppose you spend 30.0 minutes on a stair-climbing...Ch. 12 - Hydrothermal vents deep on the ocean floor spout...Ch. 12 - An electrical power plant has an overall...Ch. 12 - A diatomic ideal gas expands from a volume of VA =...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- No chatgpt pls will upvotearrow_forwardYou are standing a distance x = 1.75 m away from this mirror. The object you are looking at is y = 0.29 m from the mirror. The angle of incidence is θ = 30°. What is the exact distance from you to the image?arrow_forwardFor each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank youarrow_forward
- A planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).arrow_forwardWhat are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V) ammeter I =arrow_forwardsimple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forward
- A circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forwardAn L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forward
- Describe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forwardDiscuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forwardExplain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY