
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.15B, Problem 12.10P
Ethers are not easily differentiated by their infrared spectra, but they tend to form predictable fragments in the mass spectrum. The following compounds give similar but distinctive mass spectra.
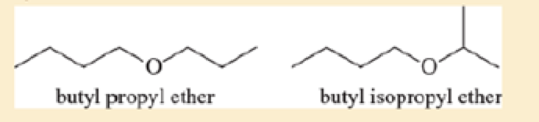
Both compounds give prominent peaks at m/z 116, 73, 57, and 43. But one compound gives a distinctive strong peak at 87, and the other compound gives a strong peak at 101. Determine which compound gives the peek at 87 and which one gives the peak at 101. Propose fragmentations to account for the ions at m/z 116, 101, 87, and 73.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Problem 7 of 10
Draw the major product of this reaction. Ignore inorganic byproducts.
S'
S
1. BuLi
2. ethylene oxide (C2H4O)
Select to Draw
a
Submit
Feedback (4/10)
30%
Retry
Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow
the arrows to draw the reactant and missing intermediates involved in this reaction.
Include all lone pairs and charges as appropriate. Ignore inorganic byproducts.
Incorrect, 6 attempts remaining
:0:
Draw the Reactant
H
H3CO
H-
HIO:
Ö-CH3
CH3OH2*
protonation
H.
a
H
(+)
H
Ο
CH3OH2
O:
H3C
protonation
CH3OH
deprotonation
>
CH3OH
nucleophilic addition
H.
HO
0:0
Draw Intermediate
a
X
Can I please get the blank spaces answered/answers?
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Identify the following alkenes as E or Z NH₂ Br 2. Draw the structures based on the IUPAC names (3R,4R)-3-bromo-4-fluoro- 1-hexene (Z)-4-bromo-2-iodo-3-ethyl- 3-heptene تر 3. For the following, predict all possible elimination product(s) and circle the major product. HO H₂SO4 Heat 80 F4 OH H2SO4 Heat 어요 F5 F6 1 A DII 4 F7 F8 F9 % & 5 6 7 * ∞ 8 BAB 3 E R T Y U 9 F D G H J K O A F11 F10arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. ○ O 1. H₂O, pyridine 2. neutralizing work-up a N W X 人 Parrow_forward✓ Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. tab OH CI 0 Br xx Br None of these molecules have a total of five ẞ hydrogens. esc Explanation Check caps lock shift 1 fn control 02 F2 W Q A N #3 S 80 F3 E $ t 01 205 % 5 F5 & 7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility FT * 8 R T Y U כ F6 9 FIG F11 F D G H J K L C X V B < N M H option command P H + F12 commandarrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts and the carboxylic acid side product. O 1. CHзMgBr (excess) 2. H₂O ✓ W X 人arrow_forwardIf cyclopentyl acetaldehyde reacts with NaOH, state the product (formula).arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. N S S HgCl2, H2SO4 く 8 W X Parrow_forward
- tab esc く Drawing the After running various experiments, you determine that the mechanism for the following reaction occurs in a step-wise fashion. Br + OH + Using this information, draw the correct mechanism in the space below. 1 Explanation Check F2 F1 @2 Q W A os lock control option T S # 3 80 F3 Br $ 4 0105 % OH2 + Br Add/Remove step X C F5 F6 6 R E T Y 29 & 7 F D G H Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce A F7 DII F8 C Ո 8 * 9 4 F10 F C J K L C V Z X B N M H command P ge Coarrow_forwardIndicate compound A that must react with ethylbenzene to obtain 4-ethylbenzene-1-sulfonic acid. 3-bromo-4-ethylbenzene-1-sulfonic acid.arrow_forwardPart 1 of 2 Draw the structure of A, the minor E1 product of the reaction. esc I Skip Part Check H₂O, D 2 A + Click and drag to start drawing a structure. -0- F1 F2 1 2 # 3 Q A 80 F3 W E S D F4 $ 4 % 5 F5 ㅇ F6 R T Y F G X 5 & 7 + Save 2025 McGraw Hill LLC. All Rights Reserved. DII F7 F8 H * C 80 J Z X C V B N 4 F9 6arrow_forward
- File Preview The following is a total synthesis of the pheromone of the western pine beetle. Such syntheses are interesting both because of the organic chemistry, and because of the possibility of using species specific insecticides, rather than broad band insecticides. Provide the reagents for each step. There is some chemistry from our most recent chapter in this synthesis, but other steps are review from earlier chapters. (8 points) COOEt COOEt A C COOEt COOEt COOH B OH OTS CN D E See the last homework set F for assistance on this one. H+, H₂O G OH OH The last step is just nucleophilic addition reactions, taking the ketone to an acetal, intramolecularly. But it is hard to visualize the three dimensional shape as it occurs. Frontalin, pheromone of the western pine beetlearrow_forwardFor the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. C Major Product: Check + ◎ + X ง © Cl I F2 80 F3 I σ F4 I F5 NaOH Click and drawing F6 A 2025 McGraw Hill LLC. All Rights E F7 F8 $ # % & 2 3 4 5 6 7 8 Q W E R T Y U A S D F G H Jarrow_forwardCan I please get help with this graph. If you can show exactly where it needs to pass through.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY