
(a)
To determine: The characteristic infrared absorptions of the
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or
(a)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
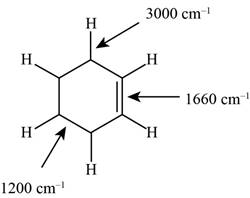
Figure 1
Explanation of Solution
The structure of the given molecule is,
Figure 2
The possibilities of the IR stretching frequencies for the given molecule are shown as,
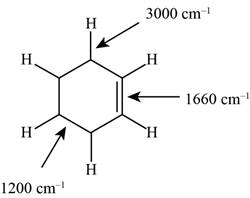
Figure 1
So, the stretching frequencies of
(b)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(b)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
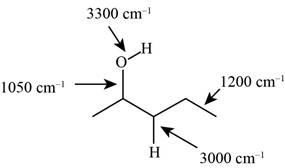
Figure 3
Explanation of Solution
The structure of the given molecule is,
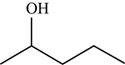
Figure 4
The possibilities of the IR stretching frequencies for the given molecule are shown as,
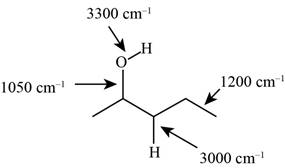
Figure 3
So, the stretching frequencies of
(c)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(c)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
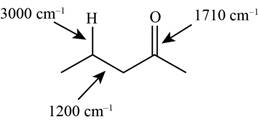
Figure 5
Explanation of Solution
The structure of the given molecule is,
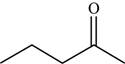
Figure 6
The possibilities of the IR stretching frequencies for the given molecule are shown as,
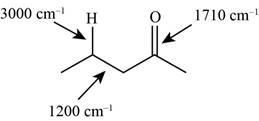
Figure 5
So, the stretching frequencies of
(d)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(d)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
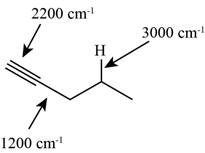
Figure 7
Explanation of Solution
The structure of the given molecule is,
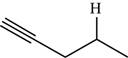
Figure 8
The possibilities of the IR stretching frequencies for the given molecule are shown as,

Figure 7
So, the stretching frequencies of
(e)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(e)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
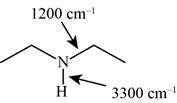
Figure 9
Explanation of Solution
The structure of the given molecule is,
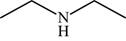
Figure 10
The possibilities of the IR stretching frequencies for the given molecule are shown as,
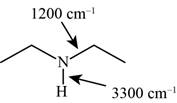
Figure 9
So, the stretching frequencies of
(f)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule is to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(f)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
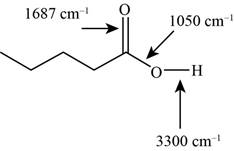
Figure 11
Explanation of Solution
The structure of the given molecule is,
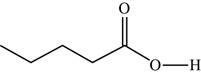
Figure 12
The possibilities of the IR stretching frequencies for the given molecule are shown as,

Figure 11
So, the stretching frequencies of
(g)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(g)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
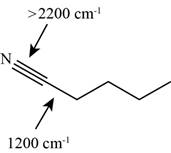
Figure 13
Explanation of Solution
The structure of the given molecule is,
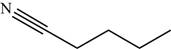
Figure 14
The possibilities of the IR stretching frequencies for the given molecule are shown as,
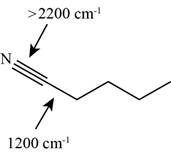
Figure 13
So, the stretching frequencies of
(h)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(h)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
Figure 15
Explanation of Solution
The structure of the given molecule is,
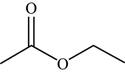
Figure 16
The possibilities of the IR stretching frequencies for the given molecule are shown as,
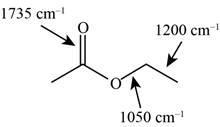
Figure 15
So, the stretching frequencies of
(i)
To determine: The characteristic infrared absorptions of the functional groups in the given molecule.
Interpretation: The characteristic infrared absorptions of the functional groups in the given molecule are to be predicted.
Concept introduction: An IR spectrum is a graph for the energy absorbed by a molecule as a function of the frequency or wavelength of light. Alkanes, alkenes and alkynes have characteristic
(i)
Answer to Problem 12.12SP
The characteristic infrared absorptions of the functional groups in the given molecule are,
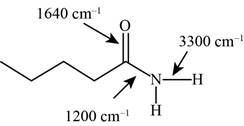
Figure 17
Explanation of Solution
The structure of the given molecule is,
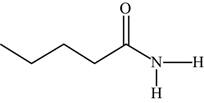
Figure 18
The possibilities of the IR stretching frequencies for the given molecule are shown as,

Figure 17
So, the stretching frequencies of
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry (9th Edition)
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

