
(a)
Interpretation:
A multistep synthesis of the given compounds from the given starting material has to be given.
Concept introduction:
Bromination:

2-methyl propane undergoes radical bromination which yields the 2-bromo-2-methylpropane.because bromination will occur where the tertiary radical is present. (bromination reactions are more selective reaction).
Formation of
The
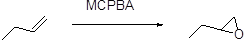
(b)
Interpretation:
A multistep synthesis of the given compounds from the given starting material has to be given.
Concept introduction:
Bromination:

2-methyl propane undergoes radical bromination which yields the 2-bromo-2-methylpropane.because bromination will occur where the tertiary radical is present. (bromination reactions are more selective reaction).
Oxidation of alcohol:
Alcohols reacts with hypochlorous (oxidizing agent) in the presence of acetic acid which yields the corresponding
Primary alcohols gives aldehyde, secondary alcohols gives ketone.
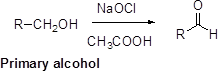
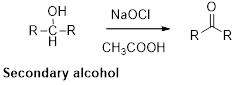
The alcohols reacts with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms which is bearing alcohol group and yields the corresponding product.
(c)
Interpretation:
A multistep synthesis of the given compounds from the given starting material has to be given.
Concept introduction:
Bromination:

2-methyl propane undergoes radical bromination which yields the 2-bromo-2-methylpropane.because bromination will occur where the tertiary radical is present. (bromination reactions are more selective reaction).
Oxidation of alcohol:
Alcohols reacts with hypochlorous (oxidizing agent) in the presence of acetic acid and yields the corresponding aldehyde and ketones.
Primary alcohols gives aldehyde, secondary alcohols gives ketone.
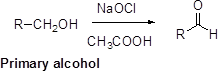
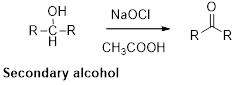
SN2 reaction:
The alcohols reacts with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms which is bearing alcohol group and yields the corresponding product.
(d)
Interpretation:
A multistep synthesis of the given compounds from the given starting material has to be given.
Concept introduction:
Bromination:

2-methyl propane undergoes radical bromination which yields the 2-bromo-2-methylpropane.because bromination will occur where the tertiary radical is present. (bromination reactions are more selective reaction).
Formation of epoxide:
The alkene can be converted to epoxide when alkene is treated with MCPBA (m-chloro perbenzoic acid)
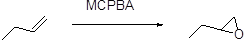
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
- pressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward
- 6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
