
Concept explainers
(a)
Interpretation:
The product for the given set of reactions should be identified.
Concept introduction:
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms. The oxidation of alcohol are achieved by using reagents like
(b)
Interpretation:
The product for the given set of reactions should be identified.
Concept introduction:
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms. The oxidation of alcohol are achieved by using reagents like
(c)
Interpretation:
The product for the given set of reactions should be identified.
Concept introduction:
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms. The oxidation of alcohol are achieved by using reagents like
(d)
Interpretation:
The product for the given set of reactions should be identified.
Concept introduction:
Swern oxidation:
Primary and secondary alcohols undergoes oxidation reaction using dimethyl sulfoxide, oxalyl chloride and triethyl
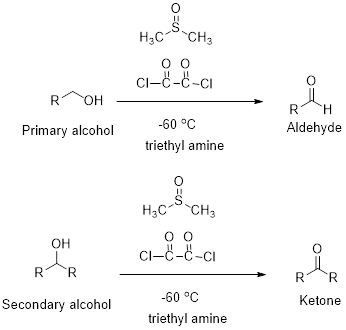
(e)
Interpretation:
The product for the given set of reactions should be identified.
Concept introduction:
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms. The oxidation of alcohol are achieved by using reagents like
(f)
Interpretation:
The product for the given set of reactions should be identified.
Concept introduction:
Oxidation Reaction: The Dess-Martin Periodinane (DMP), a hyper valent iodine compound, offers selective and very mild oxidation of alcohols to aldehydes.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





